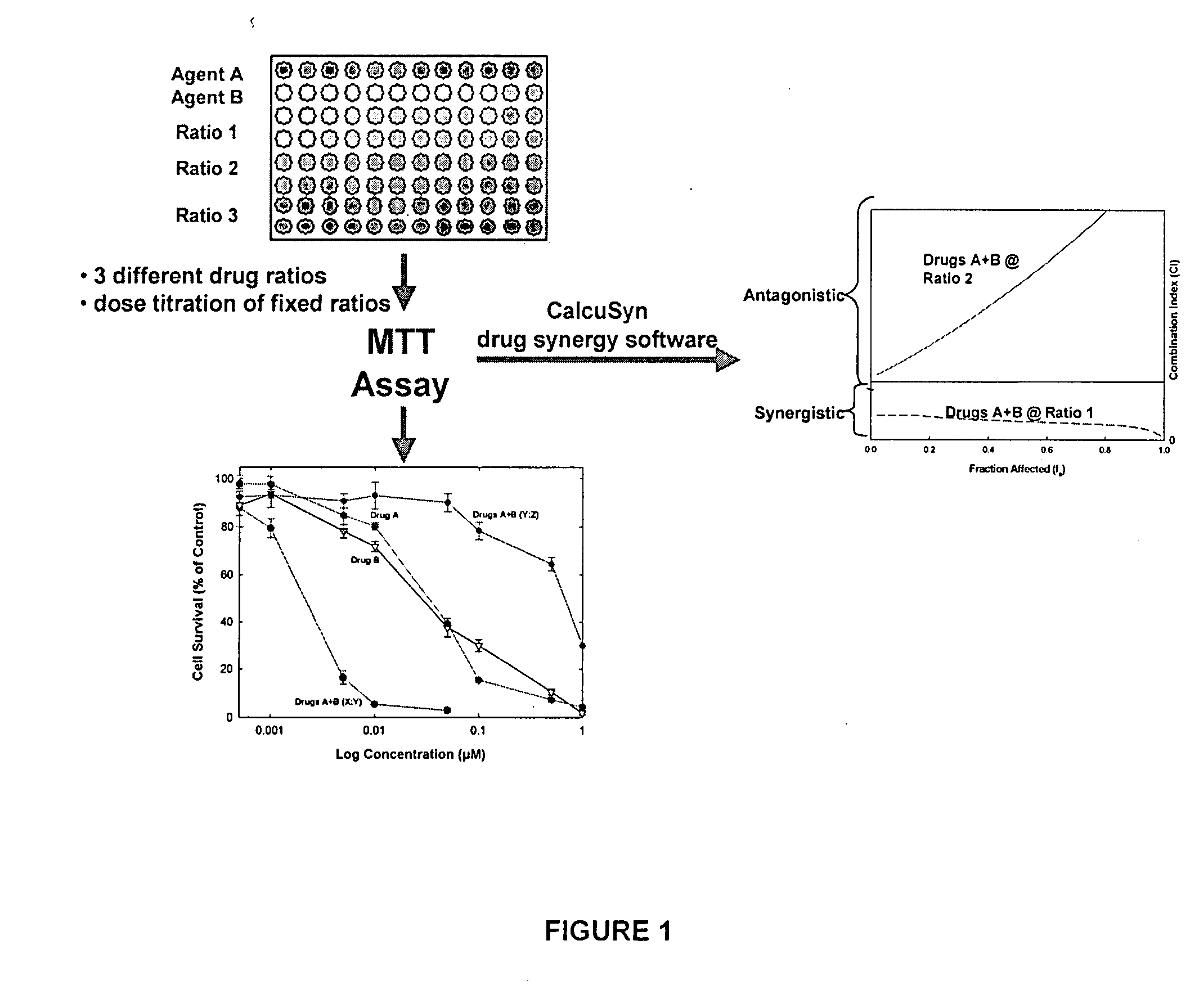
Compositions for delivery of drug combinations
a technology of compound compositions and drugs, applied in the direction of drug compositions, biocide, heterocyclic compound active ingredients, etc., can solve the problems of limited success in achieving cures with single agents, limiting their therapeutic use, and affecting the effect of the same combination of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Multiple Representation of Dose-Effect Analysis
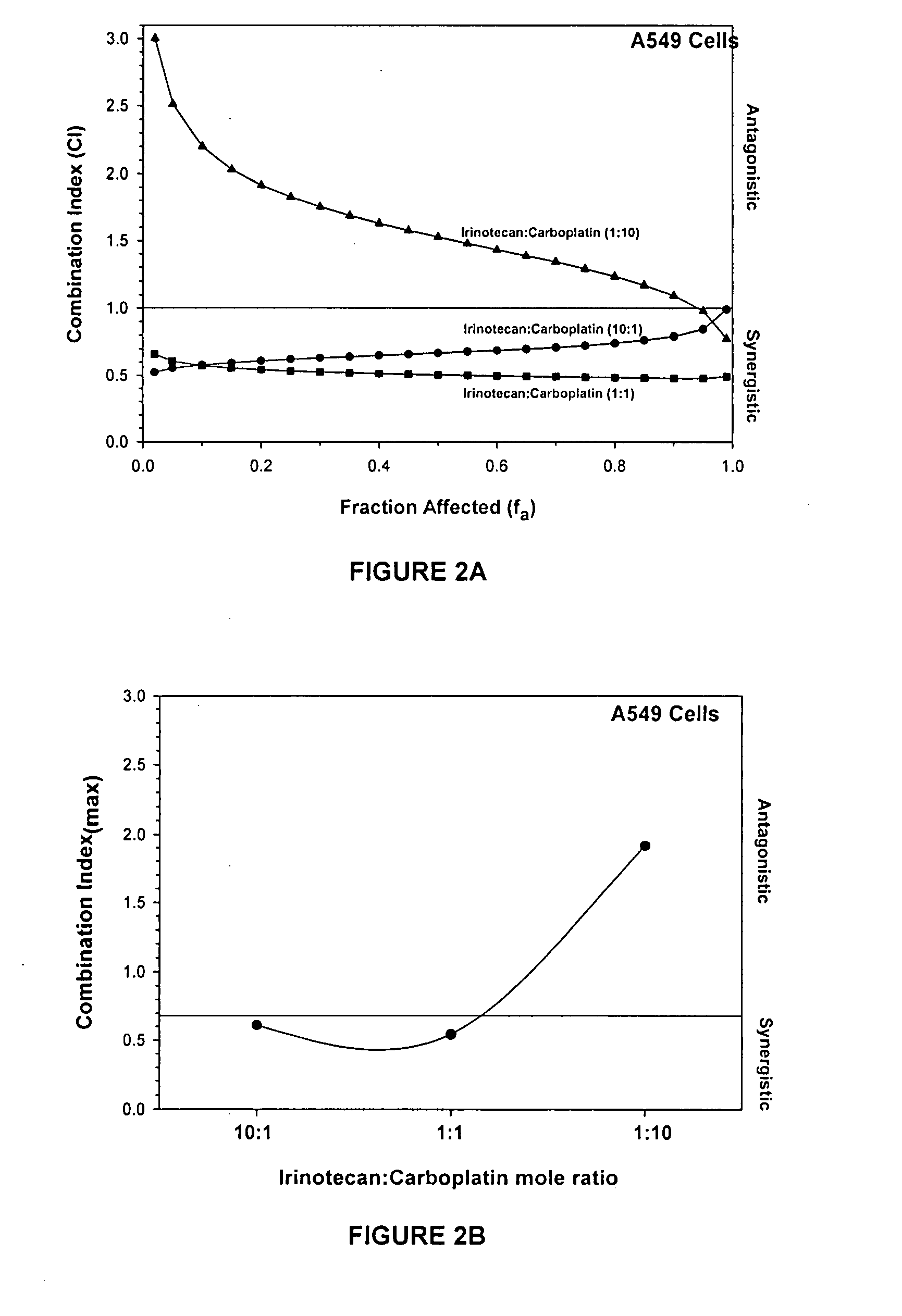
[0201] Quantitative analysis of the relationship between an amount (dose or concentration) of drug and its biological effect as well as the joint effect of drug combinations can be measured and reported in a number of ways. FIG. 2 illustrates 5 such methods using, as an example, a combination of irinotecan and carboplatin.
[0202] Based on Chou and Talalay's theory of dose-effect analysis, a “median-effect equation” has been used to calculate a number of biochemical equations that are extensively used in the art. Derivations of this equation have given rise to higher order equations such as those used to calculate Combination Index (CI). As mentioned previously, CI can be used to determine if combinations of more than one drug and various ratios of each combination are antagonistic, additive or synergistic. CI plots are typically illustrated with CI representing the y-axis versus the proportion of cells affected, or fraction affected (f...
example 2
CI is Dependent upon Concentrations
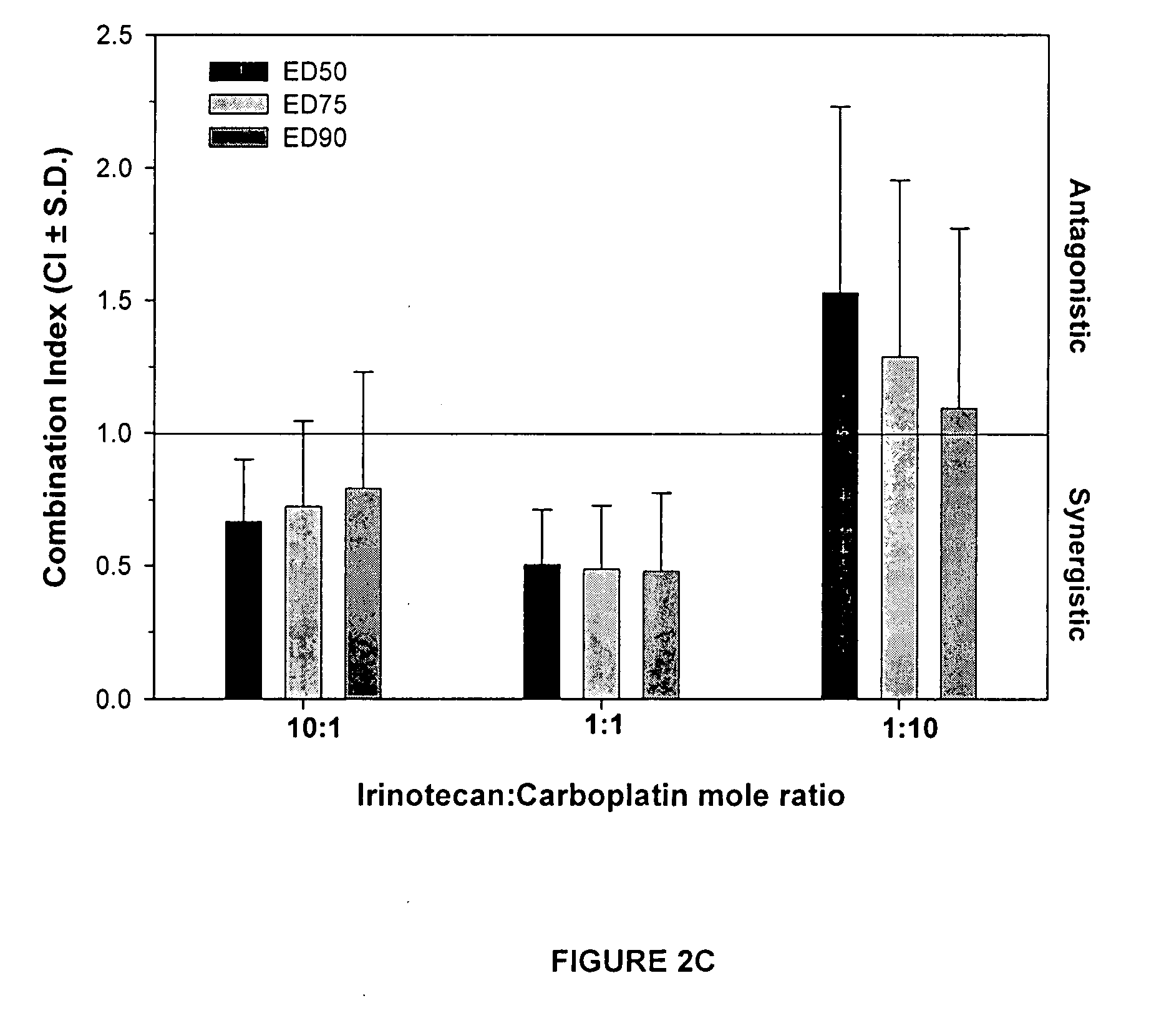
[0207] Drug combinations of irinotecan and 5-Fluorouracil (5-FU) at mole ratios of 1:1 and 1:10 and etoposide and carboplatin at mole ratios of 10:1 and 1:10 were tested for additive, synergistic or antagonistic effects using the standard tetrazolium-based calorimetric MTT cytotoxicity assay and the median-effect analysis as described in the previous example sections. HT29 or MCF-7 cells were exposed to single agents as well as agents in combination at defined ratios. Eight drug concentrations were utilized for single agents and combinations. Optical density values were obtained from the MTT assay, converted into a percentage of the control, averaged and then converted into fraction affected values. Dose and fraction affected values were entered into CalcuSyn which yielded the CI versus fa graph, shown in FIG. 3.
[0208]FIG. 3A shows that irinotecan and 5-FU at a mole ratio of 1:1 were non-antagonistic over the entire range of concentrations as mea...
example 3
Determination of CI for Various Two-Drug Combinations
[0211] Various drug combinations presented in the table below were tested for additive, synergistic or antagonistic effects using the MTT cytotoxicity assay protocol and the median-effect analysis procedure described above. Results from the CI versus fa graphs are tabulated below. The approximate percentage of the fa range that exhibited a non-antagonistic effect is reported in brackets following the ratio. Measurements were taken between fa values of 0.2 and 0.8 and the percent of that fa range exhibiting a synergistic or additive effect (non-antagonistic) was calculated by determining the percentage of the curve falling below a CI value of 1.1. Data is derived from at least one experiment performed in triplicate.
CELLMOLE RATIODRUG COMBINATIONLINE[% Synergistic or Additivea]Irinotecan:5-FUH4601:10 [83%], 1:1 [17%],10:1 [100%]Irinotecan:5-FUMCF-71:10 [48% additiveb], 1:1[58%], 10:1 [90%]Irinotecan:5-FUHT291:10 [75%], 1:1 [100%]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mole ratio | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com