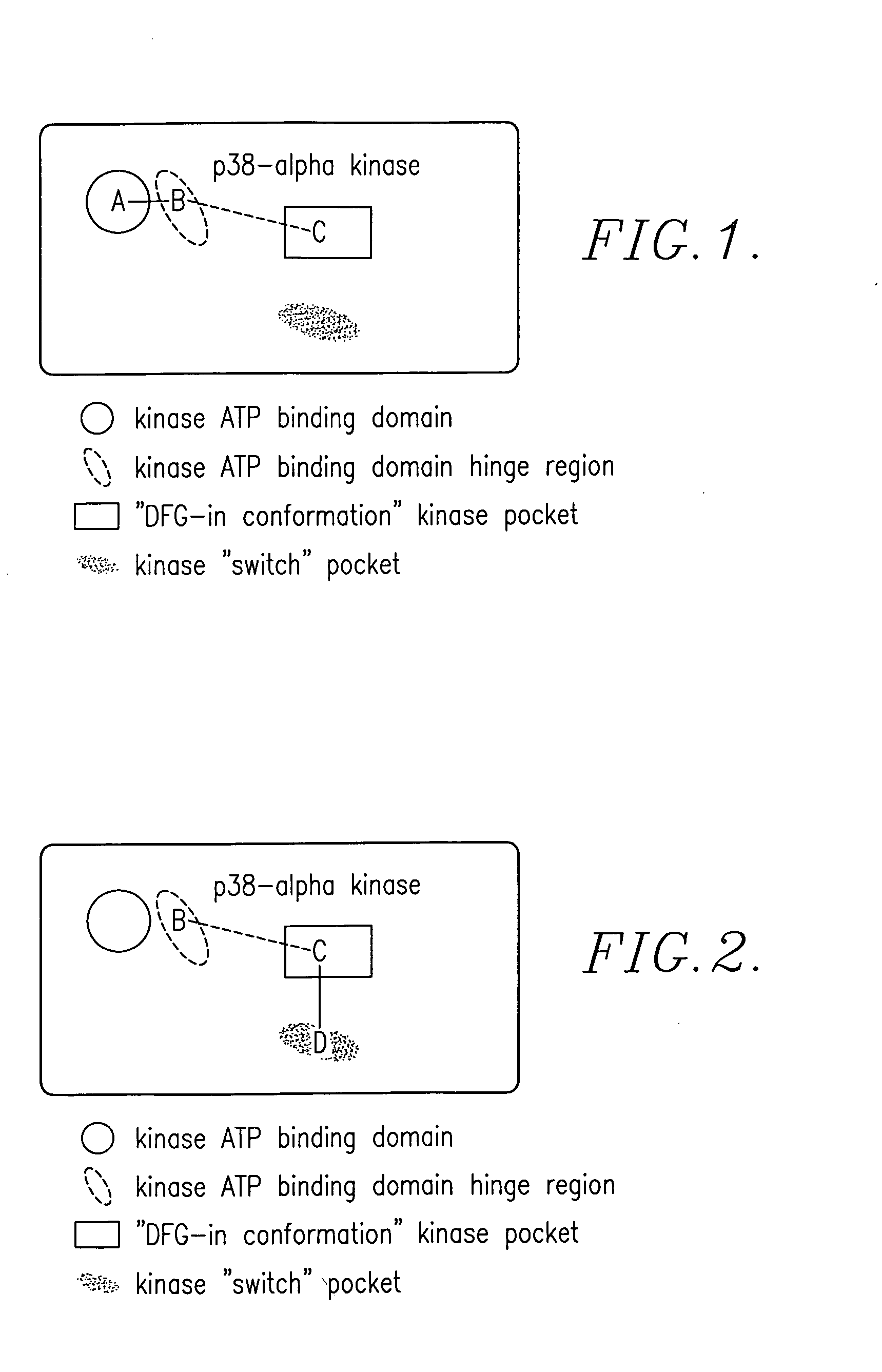
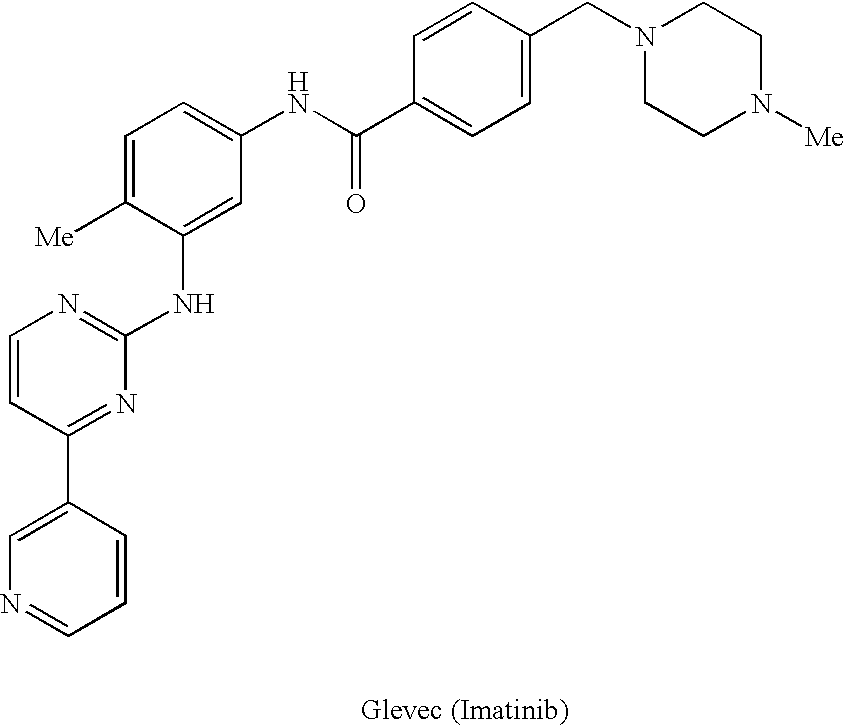
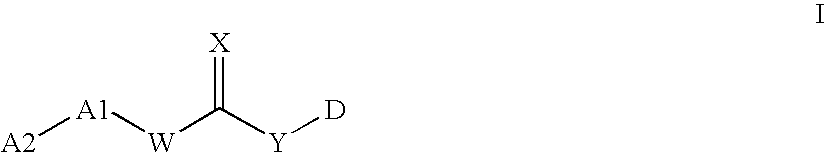Enzyme modulators and treatments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The following descriptions refer to various compounds and moieties thereof. Generally, the following definitions apply to these descriptions, with the understanding that in some instances the descriptions are further limited. However, as broadly defined, the following definitions apply.
[0018] Carbocyclyl refers to monocyclic saturated carbon rings taken from cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and cycloheptanyl;
[0019] Aryl refers to monocyclic or fused bicyclic ring systems characterized by delocalized π electrons (aromaticity) shared among the ring carbon atoms of at least one carbocyclic ring; preferred aryl rings are taken from phenyl, naphthyl, tetrahydronaphthyl, indenyl, and indanyl;
[0020] Heteroaryl refers to monocyclic or fused bicyclic ring systems characterized by delocalized π electrons (aromaticity) shared among the ring carbon or heteroatoms including nitrogen, oxygen, or sulfur of at least one carbocyclic or heterocyclic ring; heteroaryl rings a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com