Coatings with crystallized active agent(s) and methods
a technology of active agents and crystallized active agents, applied in the field of coating compositions, can solve the problems of maintaining an inventory and the cost of maintaining an inventory, and achieve the effects of enhancing the formation of active agent crystals, increasing the rate of active agent nucleation, and enhancing the crystallization of active agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Coating Solutions Saturated / Unsaturated
[0103] Coating solutions were prepared with various concentrations of drug, polymers, and solvents. Specifically, five different coating solutions were prepared as follows (the coating solutions are summarized in Table 1 below):
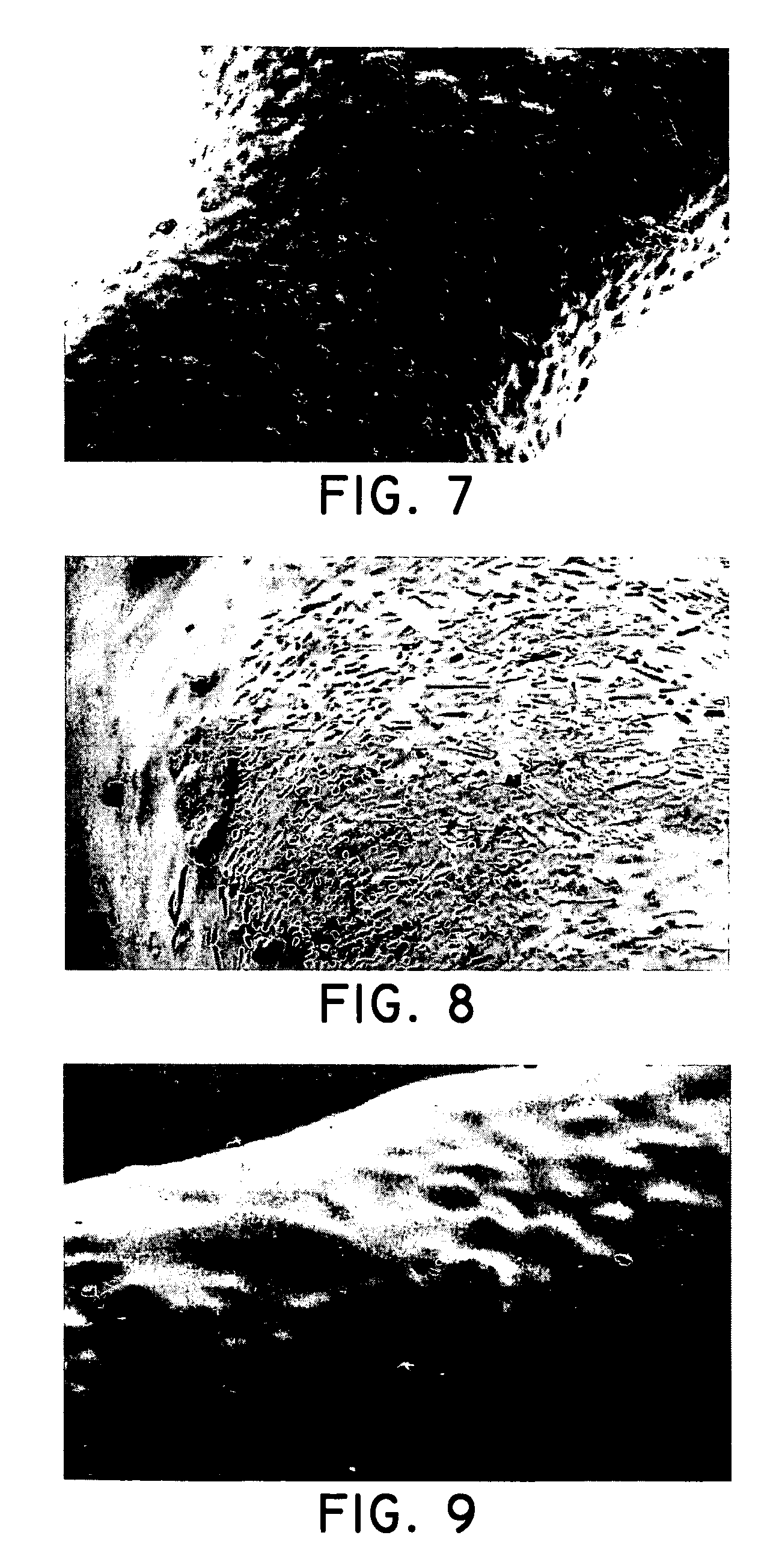
[0104] Solution 1: Estradiol was combined with THF (tetrahydrofuran) to form an active agent solution. PEVA (polyethylene-co-vinyl acetate, 33% vinyl acetate) and PBMA (poly-n-butyl methacrylate) were combined with toluene to form a polymer solution. The active agent solution and the toluene solution were combined to form a coating solution having 40 mg / ml total solids including 30 wt. % estradiol, 20 wt. % PEVA, and 50 wt. % PBMA in a solvent of 80% toluene and 20% THF (four parts toluene to one part THF). The coating solution was allowed to stand for a period of minutes at ambient temperature and was observed to be clear, indicating that the estradiol was at a soluble concentration for this solvent com...
example 2
Non-Saturated Coating Composition with THF / IPA Solvent
[0110] Estradiol, polyethylene-co-vinyl acetate (PEVA) (33% vinyl acetate), and poly-n-butyl methacrylate (PBMA) were combined in equal weight proportions in a solution that was 90% tetrahydrofuran (THF) and 10% isopropyl alcohol (IPA). The resulting solution had a total solids concentration of 40 mg / ml (33% PEVA / 33% PBMA / 33% estradiol). The resulting solution was below the saturation point for estradiol in a solvent of 90% THF / 10% IPA at ambient temperature (approximately 21-22° C.).
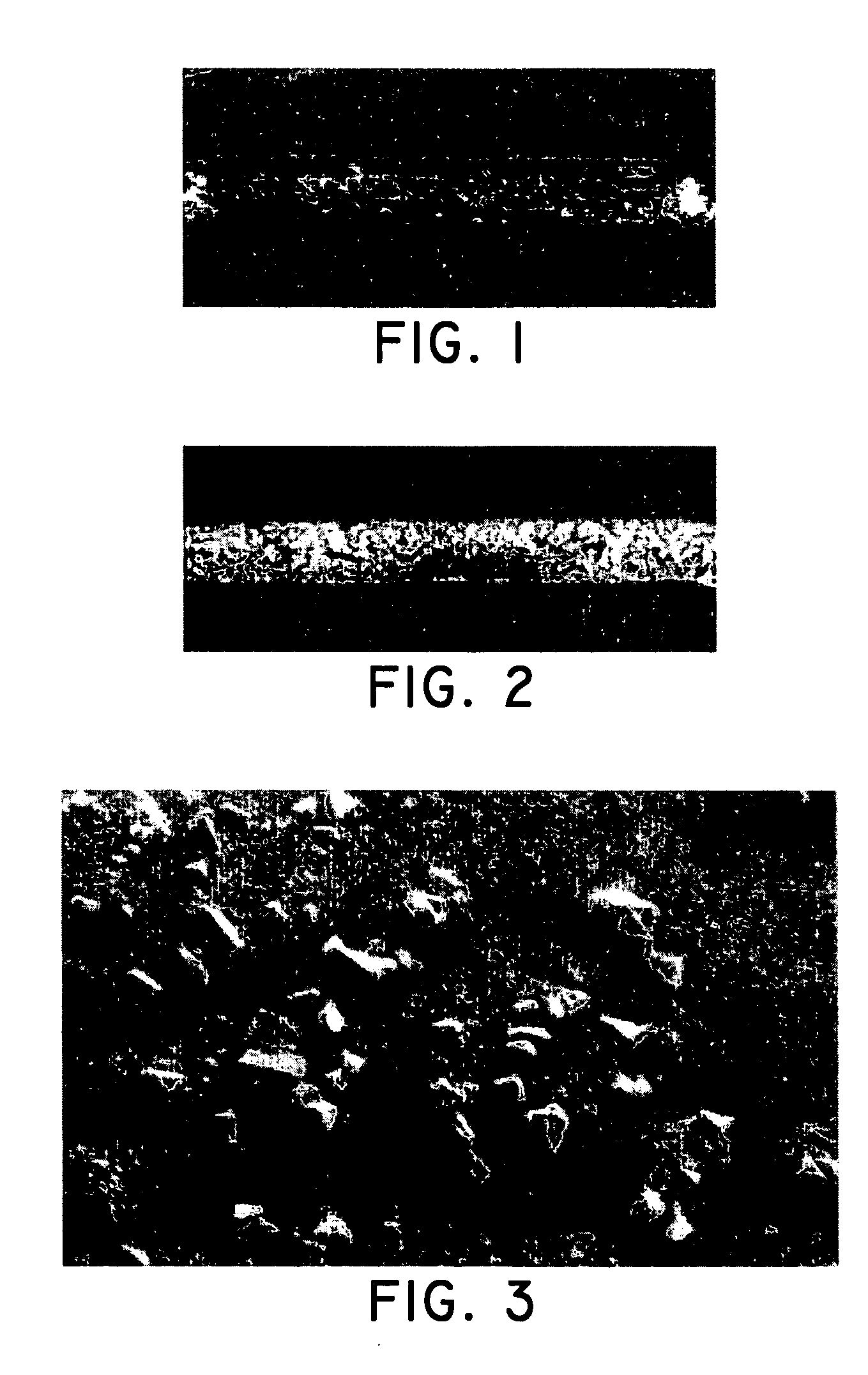
[0111] A stainless steel stent 18 mm in length was obtained and prepared by first applying a layer of parylene C using a vapor-deposition technique. After the parylene was disposed onto the stent, the coating solution was applied to the stent using an ultrasonic spray technique at a relative humidity of 10%. Ultrasonic spray techniques are disclosed in U.S. Published Application 2004 / 0062875 (Chappa et al.) the contents of which are herein incorpor...
example 3
Non-Saturated Coating Composition with Chloroform / Methanol Solvent
[0115] Estradiol, polyethylene-co-vinyl acetate (PEVA) (33% vinyl acetate), and poly-n-butyl methacrylate (PBMA) were combined in equal weight proportions in a solution that was 80% chloroform and 20% methanol. The resulting solution had a total solids concentration of 40 mg / ml (33% PEVA / 33% PBMA / 33% estradiol). The resulting solution was below the saturation point for estradiol in a solvent of 80% chloroform / 20% methanol at ambient temperature (approximately 21-22° C.).
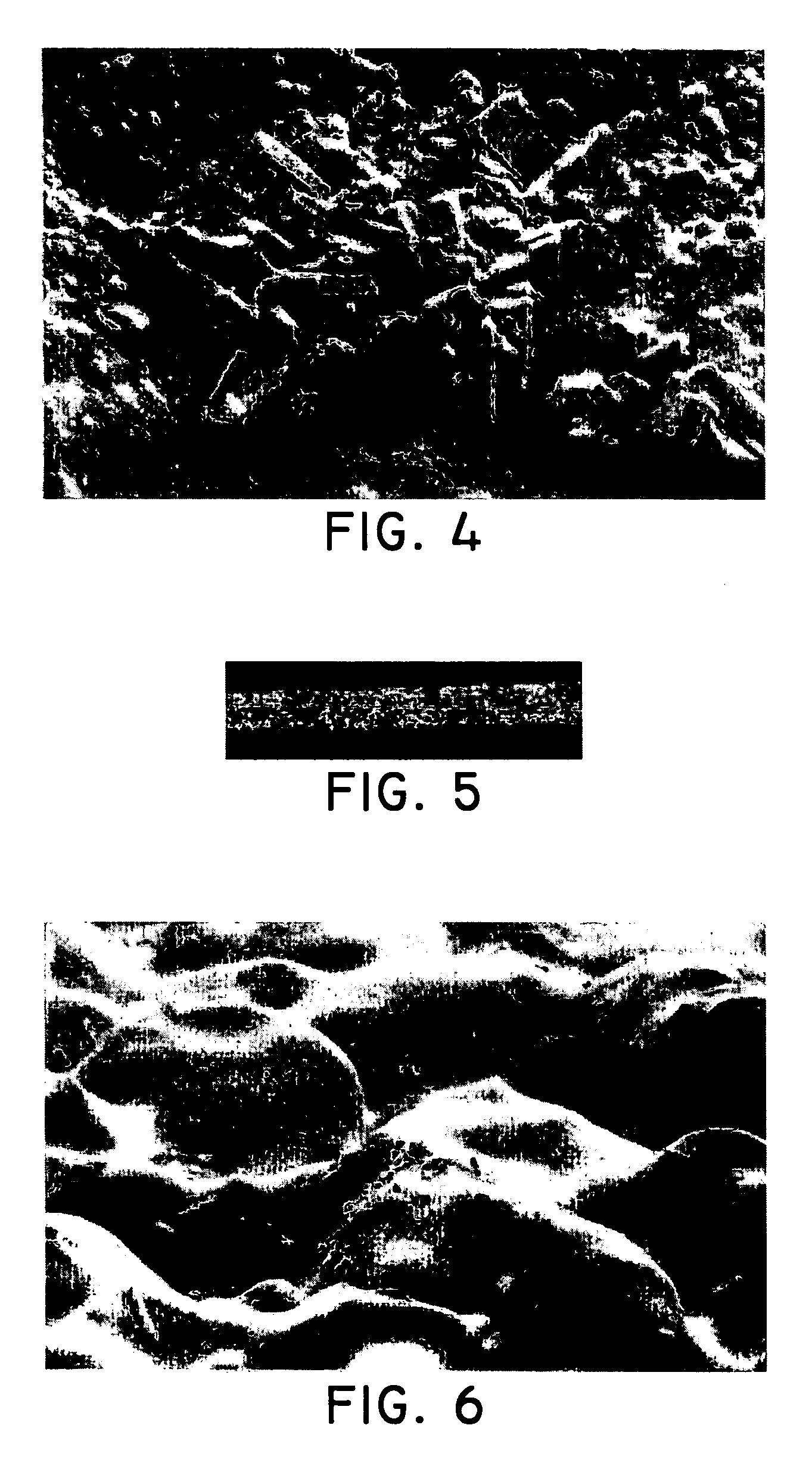
[0116] A stainless steel stent 18 mm in length was obtained and prepared by first applying a layer of parylene C using a vapor-deposition technique. After the parylene was disposed onto the stent, the coating solution was applied to the stent using an ultrasonic spray technique. A total coating weight of 683 μg was applied to the stent (measured after the solvent had substantially evaporated off) resulting in a drug loading of approximately 225 μg of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com