Iscom preparation and use thereof
a technology of iscom and preparation, applied in the field of iscom preparation, can solve the problems of prone to fever and local side effects, and achieve the effects of less toxic, less toxic, and easy tailoring of inmunogenicity or immune modulating properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Quillaja Saponaria Molina subfragment saponins
[0051] Purification of crude Quillaja Saponaria Molina extract to fractions A, B and C. A solution (0.5 ml) of crude Quillaja bark extract in water (0.5 g / ml) is pre-treated on a sep-pak column (Waters Associates, Massachusetts.).
[0052] The pre-treatment involves washing of the loaded sep-pak column with 10% acetonitrile in acidic water in order to remove hydrophilic substances. Lipophilic substances including QH-A, QH-B and QH-C are then eluted by 70% acetonitrile in water.
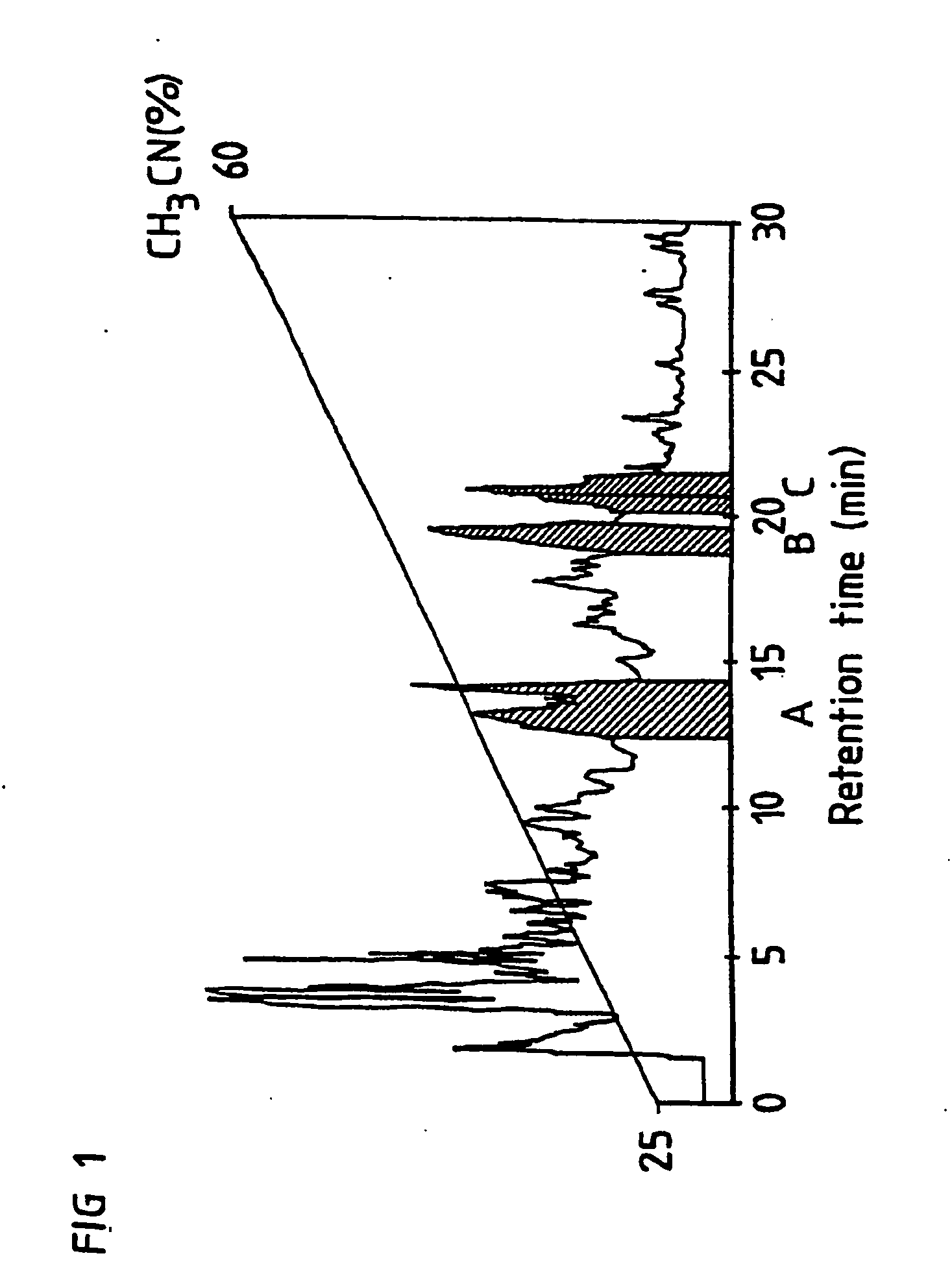
[0053] The lipophilic fraction from the sep-pak column is then separated by a semipreparative HPLC column (CT-sil, C8, 10×250 mm, ChromTech, Sweden). The sample is eluted through the column by a gradient from 25% to 60% acetonitrile in acidic water. Three fractions are collected from the HPLC column during the separation. The residues after evaporation of these three fractions constitute QH-A, QH-B and QH-C.
[0054] The fractions designated QH-A, QH-B...
example 2
Prepartion of iscom matrix
[0055] Materials
[0056] Cholesterol, e.g., Sigma C 8503)
[0057] Phosphatidyl choline (egg derived) e.g., Sigma P 3556
[0058] MEGA-10 (Bachem AG, Switzerland)
[0059]Quillaja saponin fractions A and C (patent WO9611711)
[0060] 0.22 μm Sterile filters (Acrodisc)
[0061] PBS (10 mM phosphate buffered 150 mM saline, pH 6,8-7,4)
[0062] Slide-A-Lyzer casettes MW cut off 12-14.000 (Pierce)
[0063] MEGA-10 (stock solution)
[0064] Make a 20% (w / w) stock solution by adding 8 ml distilled water to 2.0 g of dry solid MEGA-10. Dissolve by gentle heating (30-50° C.). Filter through a 0.22 nm sterile filter, aliquot and store at −20° C.
[0065] Lipid mixture (15 mg / ml)
[0066] Dissolve 100 mg of each cholesterol and phosphatidyl choline in 10 ml 20% MEGA-10. The lipids dissolve slowly at 30-60° C. with slow stirring. Filter through a 0.22 nm sterile filter, aliquot and store at −20° C. After freezing, the lipid mixtures need to be heated up to 40° C. until clear. Temperate al...
example 3
Preparation of PR-8 protein micelles
[0080] 1 Dilute 12 mg of PR-8 monomers (1.5 mg / ml) with PBS to a final protein concentration of 1.0 mg / ml.
[0081] 2 Filter through 0.22 nm sterile filter
[0082] 3 Fill into Slide-A-lyzer
[0083] 4 Dialyse against 4 changes of 2 litres of PBS (24±1° C.) (for 48-60 hours)
[0084] 5 Aspirate from Slide-A-Lyzer
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| reversed phase HPLC | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com