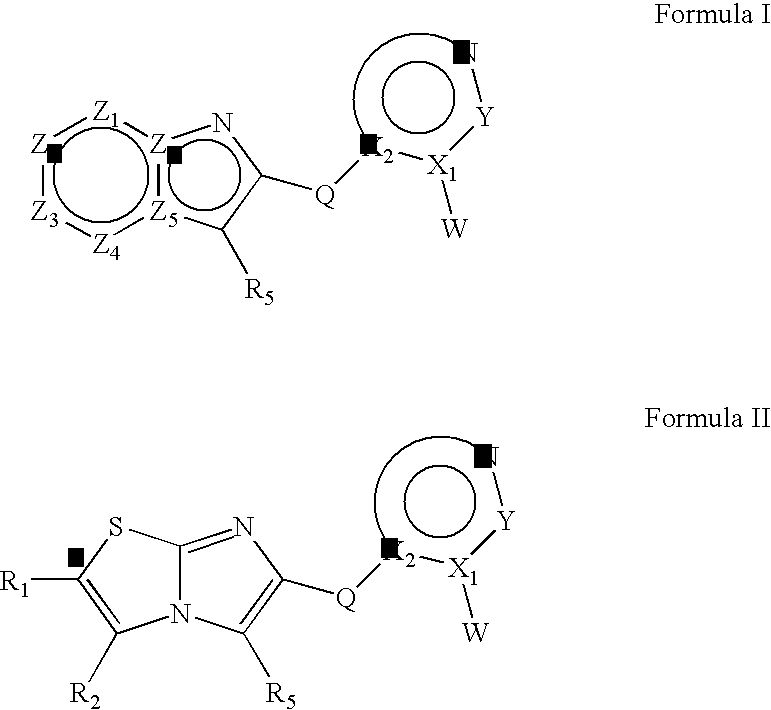
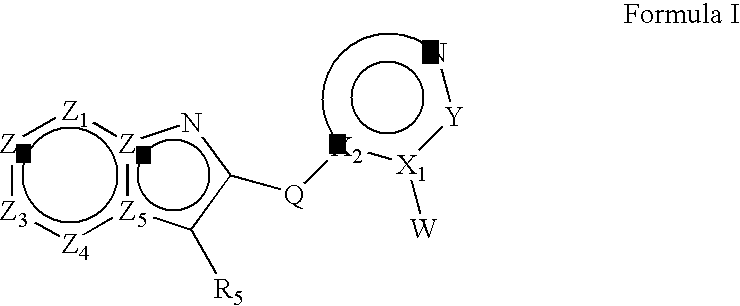
Heteroaryl substituted fused bicyclic heteroaryl compounds as GABAA receptor ligands
a technology of heteroaryl compounds and gabaa receptors, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of compound known to exhibit a number of unwanted side effects, and achieve high selectivity and affinity to
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
SYNTHESIS OF 3-FLUORO-(1H-IMIDAZOL-2-YL)BENZENE
[0195]
[0196] Saturated ammonium hydroxide solution (30 mL) is slowly added to a solution of 3-fluorobenzaldehyde (12.4 g, 100 mmol) and glyoxal (17.5 mL of 40% wt in water, 120 mmol) in methanol (100 mL) at ambient temperature. After stirring for 24 hours, most of the solvent is removed at reduced pressure. Benzene is added and evaporated to remove residual water. The resulting dark oil is purified by chromatography on silica gel (2% MeOH / CH2Cl2) to obtain a tan solid. Trituration with ether / hexane provides 3-fluoro-(1H-imidazol-2-yl)benzene as a white solid. LRMS m / z (M+1) 163.2.
example 2
SYNTHESIS OF 3-CHLORO-4-FLUORO-(1H-IMIDAZOL-2-YL)BENZENE
[0197]
[0198] A mixture of 3-chloro-4-fluoro-benzaldehyde (0.032 mol), glyoxal (40% in water, 0.038 mol) and ammonium hydroxide (28% in water, 0.16 mol) in MeOH (60 mL) is stirred at room temperature overnight. Solvent is removed in vacuo and the residue is partitioned between water and CH2Cl2. The organic layer is washed with brine, dried (Na2SO4), and concentrated. The residue is purified by column chromatography on silica gel eluting with CH2Cl2 / MeOH (95 / 5) to afford 3-chloro-4-fluoro-(1H-imidazol-2-yl)benzene as a yellow solid. 1H NMR (CDCl3) δ 7.88 (dd, 1H), 7.70 (m, 1H), 7.19 (t, 1H), 7.17 (s, 2H). LRMS m / z (M+1) 197.0.
example 3
SYNTHESIS OF 2,3,4-TRIFLUORO-(1H-IMIDAZOL-2-YL)BENZENE
[0199]
[0200] Saturated ammonium hydroxide solution (26 mL) is slowly added to a solution of 2,3,4-trifluorobenzaldehyde (5.0 g, 31.2 mmol) and glyoxal (10.75 mL of 40% wt in water, 93.7 mmol) in methanol (100 mL) at ambient temperature. After stirring for 24 hours, most of the solvent is removed at reduced pressure. Benzene is added and evaporated to remove residual water. The resulting dark oil is purified by chromatography on silica gel (5% MeOH / CH2Cl2) to obtain a tan solid. Trituration with ether / hexane provides 2,3,4-trifluoro-(1H-imidazol-2-yl)benzene as a white solid. LRMS m / z (M+1) 199.10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com