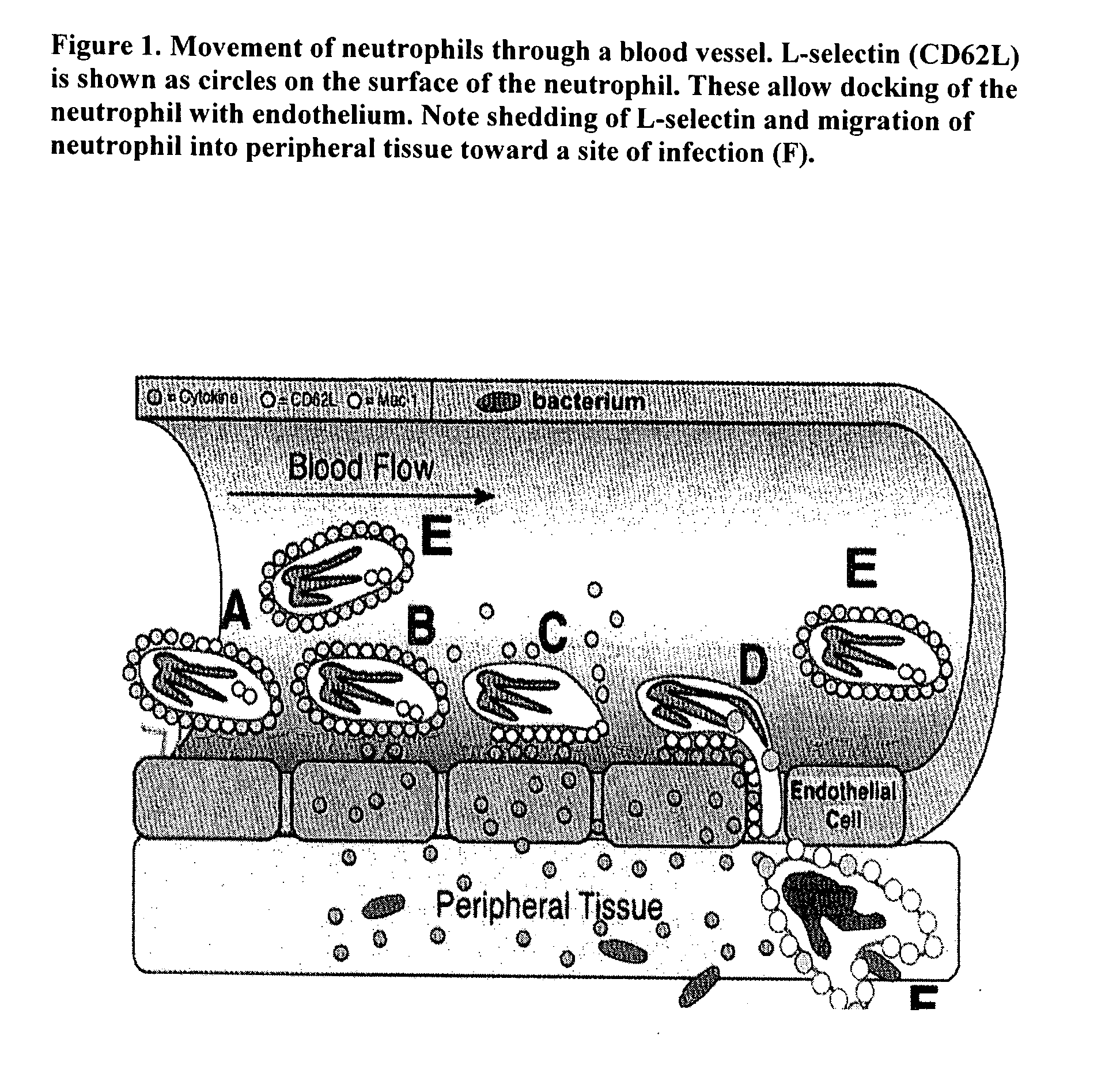
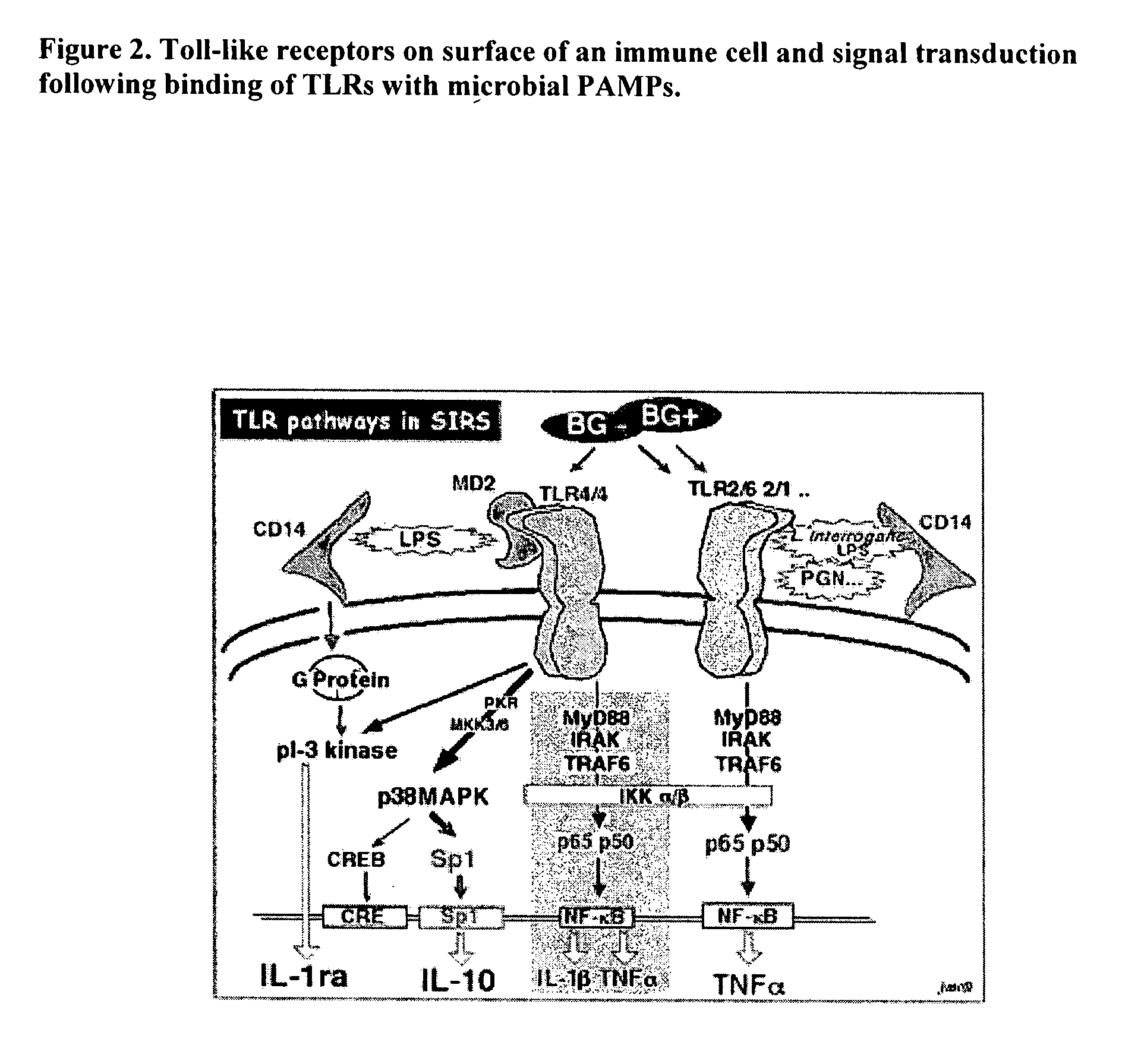
Use of beta-1,3 (4)-endoglucanohydrolase, beta-1,3 (4) glucan, diatomaceous earth, mineral clay and glucomannan to augment immune function
a technology of endoglucanohydrolase and glucan, which is applied in the field of augmentation of immune function, can solve the problems of reducing individuals' ability to fight diseases, and achieve the effects of reducing colonization by pathogens, enhancing immune function, and minimizing or eliminating invasion of blood compartments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0056] An experiment was conducted using 60 growing male and female sheep. Sheep were allocated to one of the five treatments (seven females and five males per treatment): [0057] 1. Control. [0058] 2. Immunosuppressed (daily injections of Azium [Dexamethasone], 0.1 mg / kg twice / day). [0059] 3. Immunosuppressed plus the invention fed at 0.5% of daily dry matter intake. [0060] 4. Immunosuppressed plus moldy feed (addition of Aspergillus fumigatus-infected wheat mill run; 1.5 lbs / head / day). [0061] 5. Immunosuppressed plus moldy feed (as in Treatment 4) plus the invention as outlined in Treatment 3.
[0062] Animals were fed a dairy-type diet for a period of 28 days. Immunosuppression was mediated in Treatments 2, 3, 4 and 5 by daily injection of Azium using a high dose (a model of extreme stress: Weber et al., 2001). Sheep on Treatments 4 and 5 were challenged with a pathogenic mold by feeding wheat mill run which had been contaminated with a pathogenic mold (Aspergillus fumigatus). Sheep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com