Surgical probe for supporting inflatable therapeutic devices in contact with tissue in or around body orifices and within tumors
a technology of surgical probes and therapeutic devices, which is applied in the field of surgical probes, can solve the problems of affecting the normal activation of the left and right atria, and suffering consequences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0041] The following is a detailed description of the best presently known modes of carrying out the inventions. This description is not to be taken in a limiting sense, but is made merely for the purpose of illustrating the general principles of the inventions.
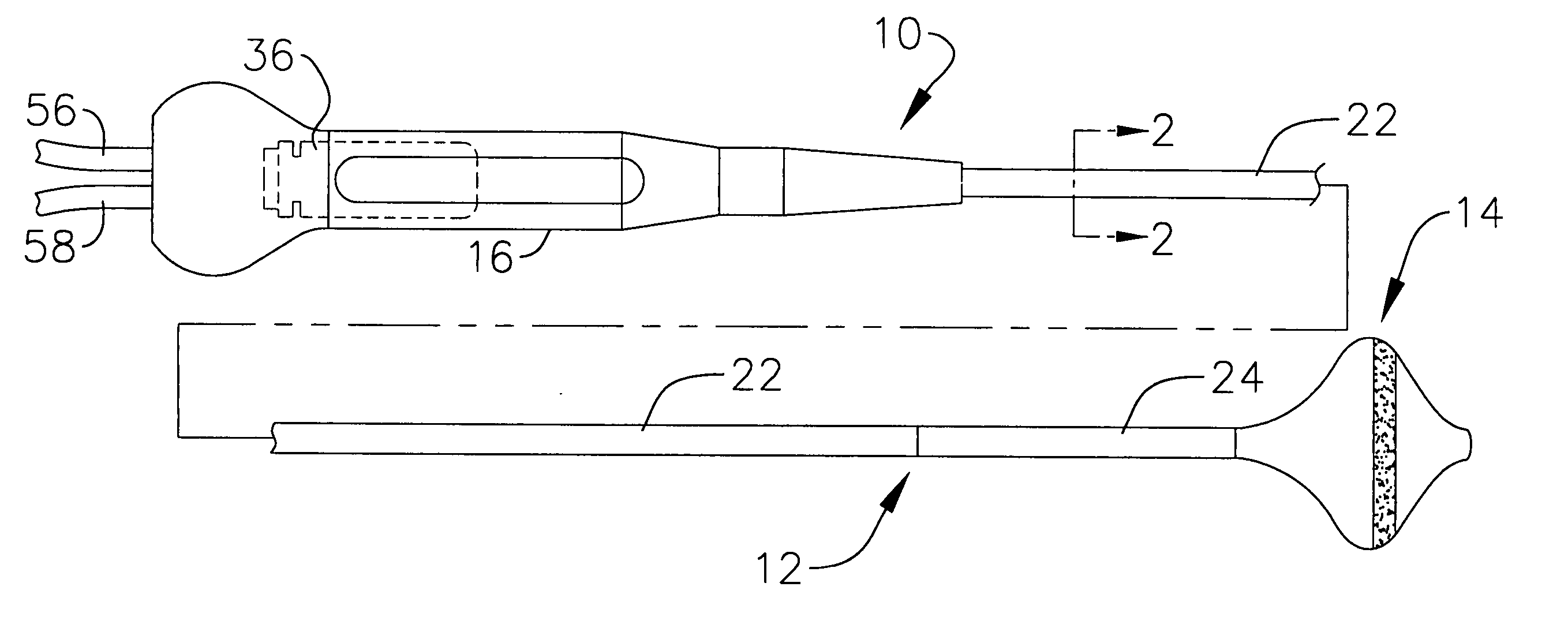
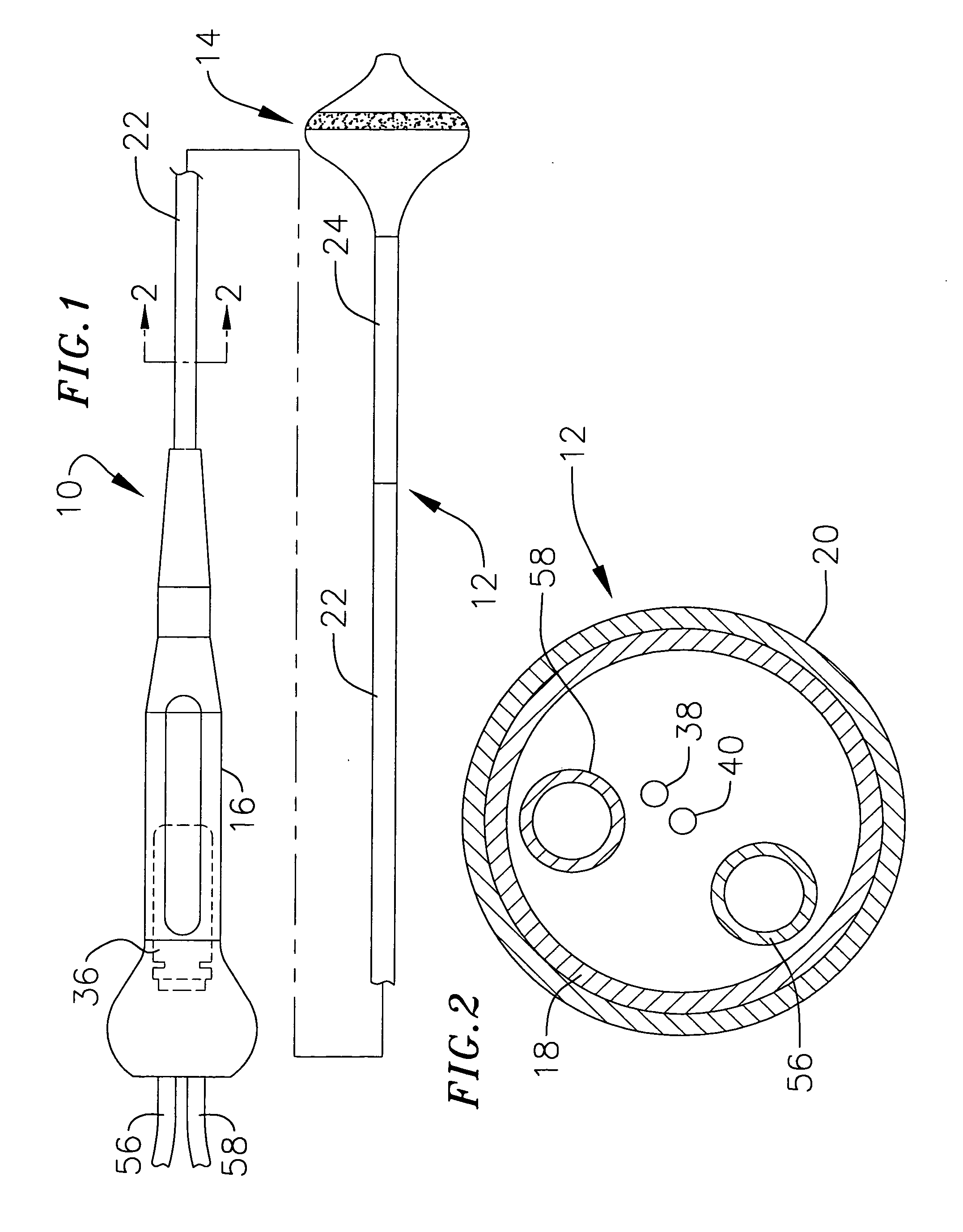
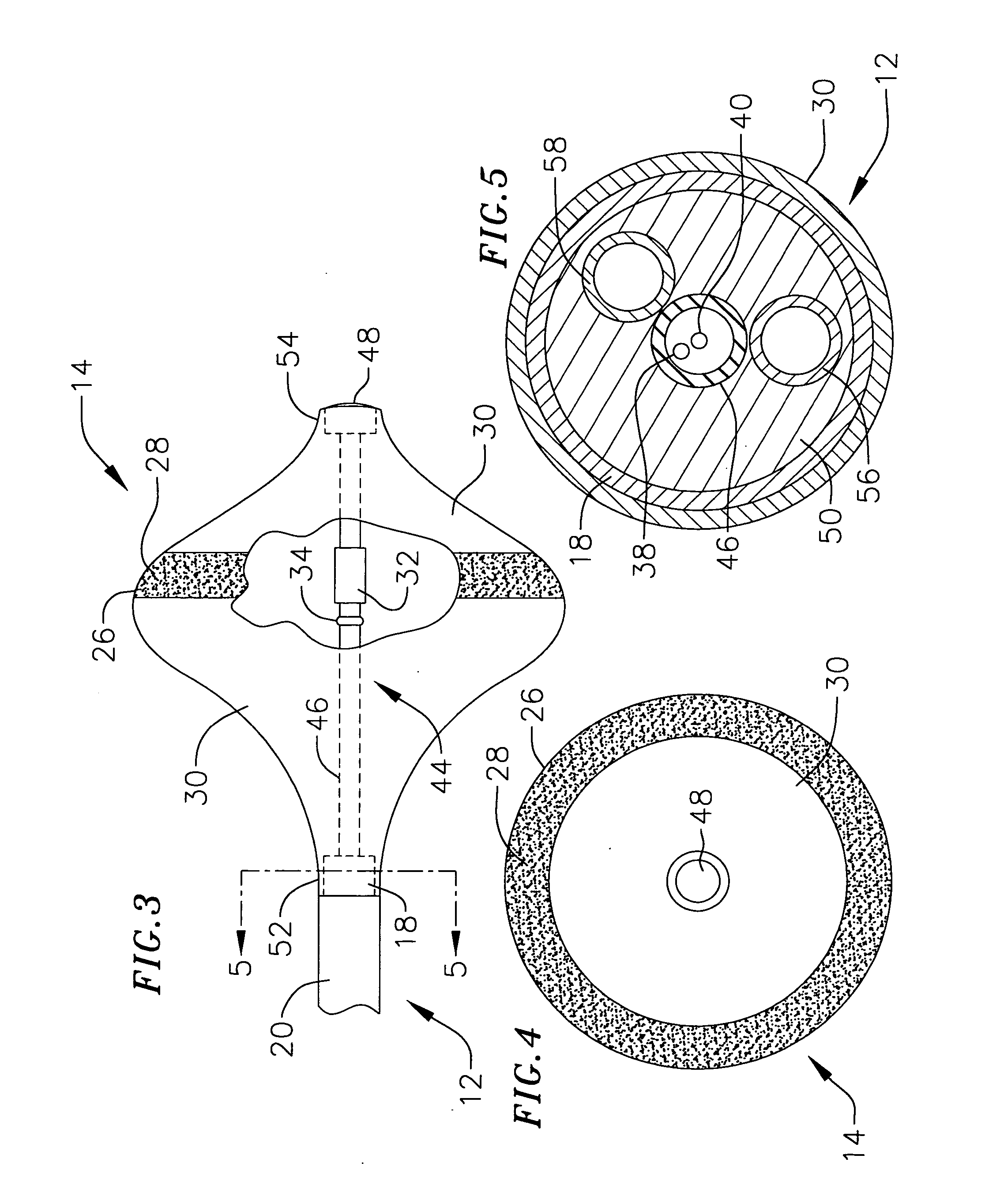
[0042] This specification discloses a number of probe structures, mainly in the context of cardiac ablation, because the structures are well suited for use with myocardial tissue. For example, the present inventions are designed to produce intimate tissue contact with target substrates associated with arrhythmias such as atrial fibrillation. One application is the creation of lesions within or around the pulmonary vein to treat ectopic atrial fibrillation. Nevertheless, it should be appreciated that the structures are applicable for use in therapies involving other types of soft tissue. For example, various aspects of the present inventions have applications in procedures concerning other regions of the body such as the pros...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com