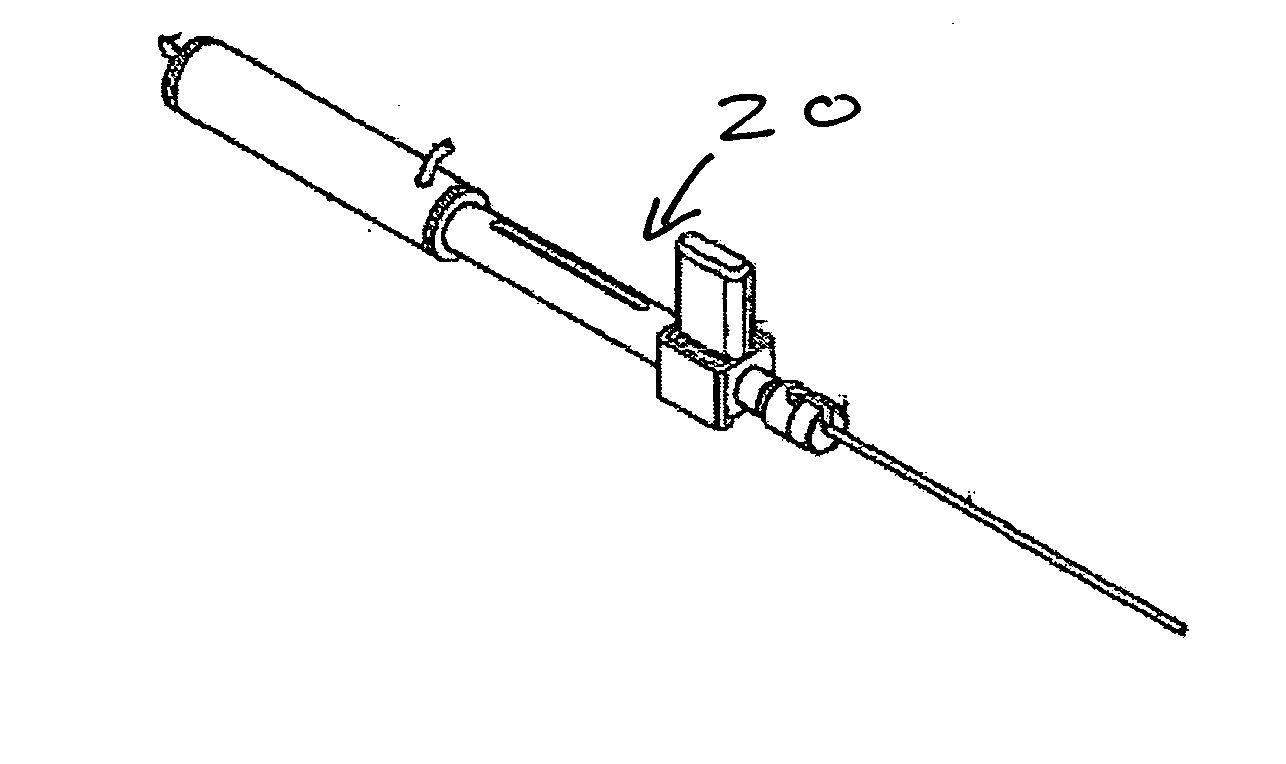
Kit for treating bony defects
a technology for bony defects and kits, applied in the field of kits for treating bony defects, can solve the problems of significant post-operative pain, potential for additional medical complications, and the volume of autograft material available from the patient's hip may not be sufficient for the graft procedure,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] The allograft mixture may generally be comprised of three components: non-demineralized cortical cancellous allograft granules or other suitable osteoconductive material, demineralized bone matrix (“DBM”) or other suitable osteoinductive material and sodium hyaluronan (HA), or other suitable lubricating carrier. The non-demineralized cortical cancellous allograft granules may generally be 200-2000 microns in size and may have an aspect ratio of about 1.5 longer than wide. The DBM may generally be 100-1000 microns in size and tends to be more uniform and rounded in shape. The lubricating carrier may generally be a viscous liquid, for example, sodium hyaluronan in varying molecular weights, alginate, dextran, gelatin, collagen and others. The DBM is more likely than the non-demineralized granules to be suspended in the lubricating carrier due to the geometric and size difference between the DBM and the non-demineralized granules.
[0013] Ceramic materials may be added as alterna...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com