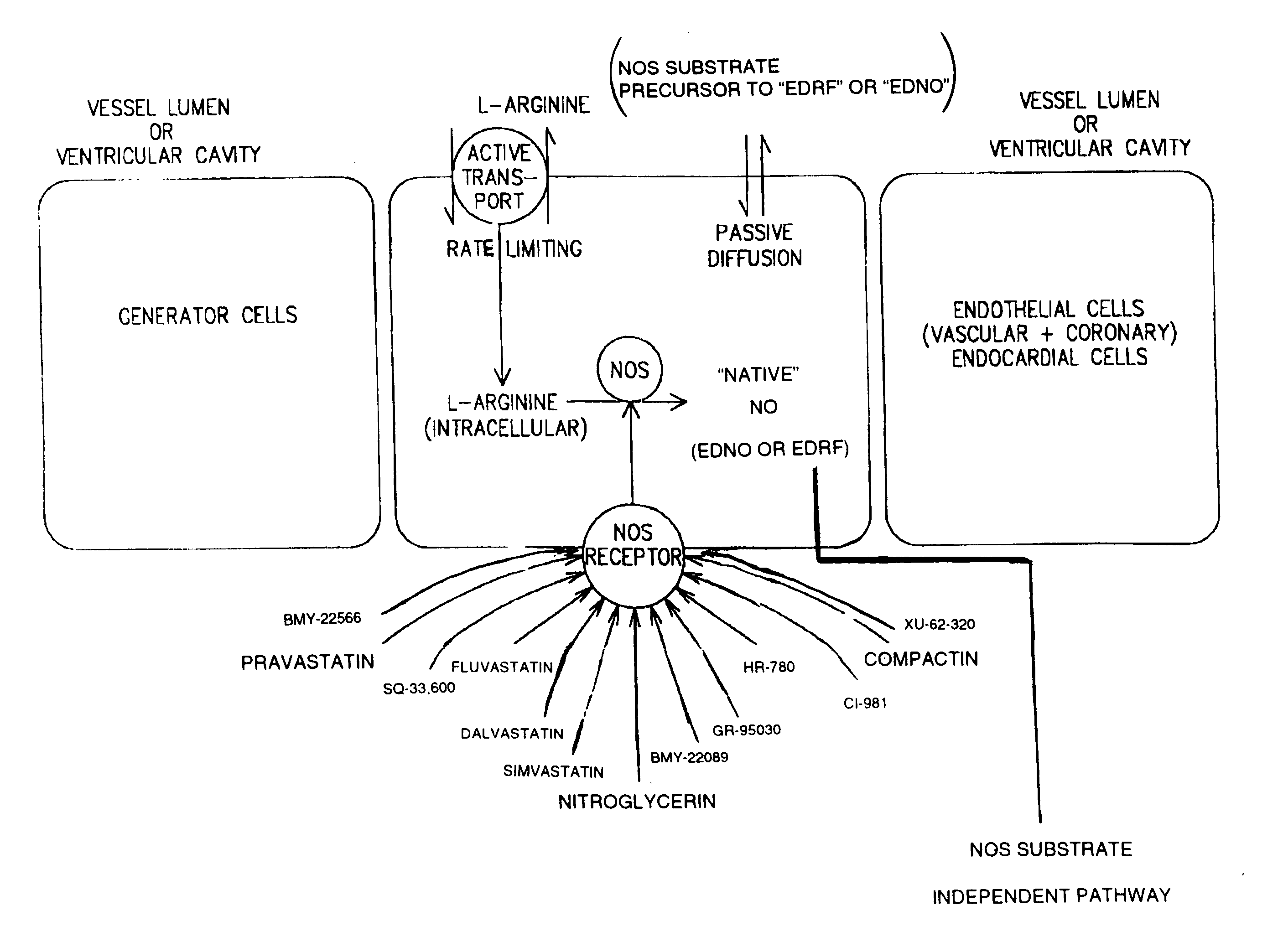
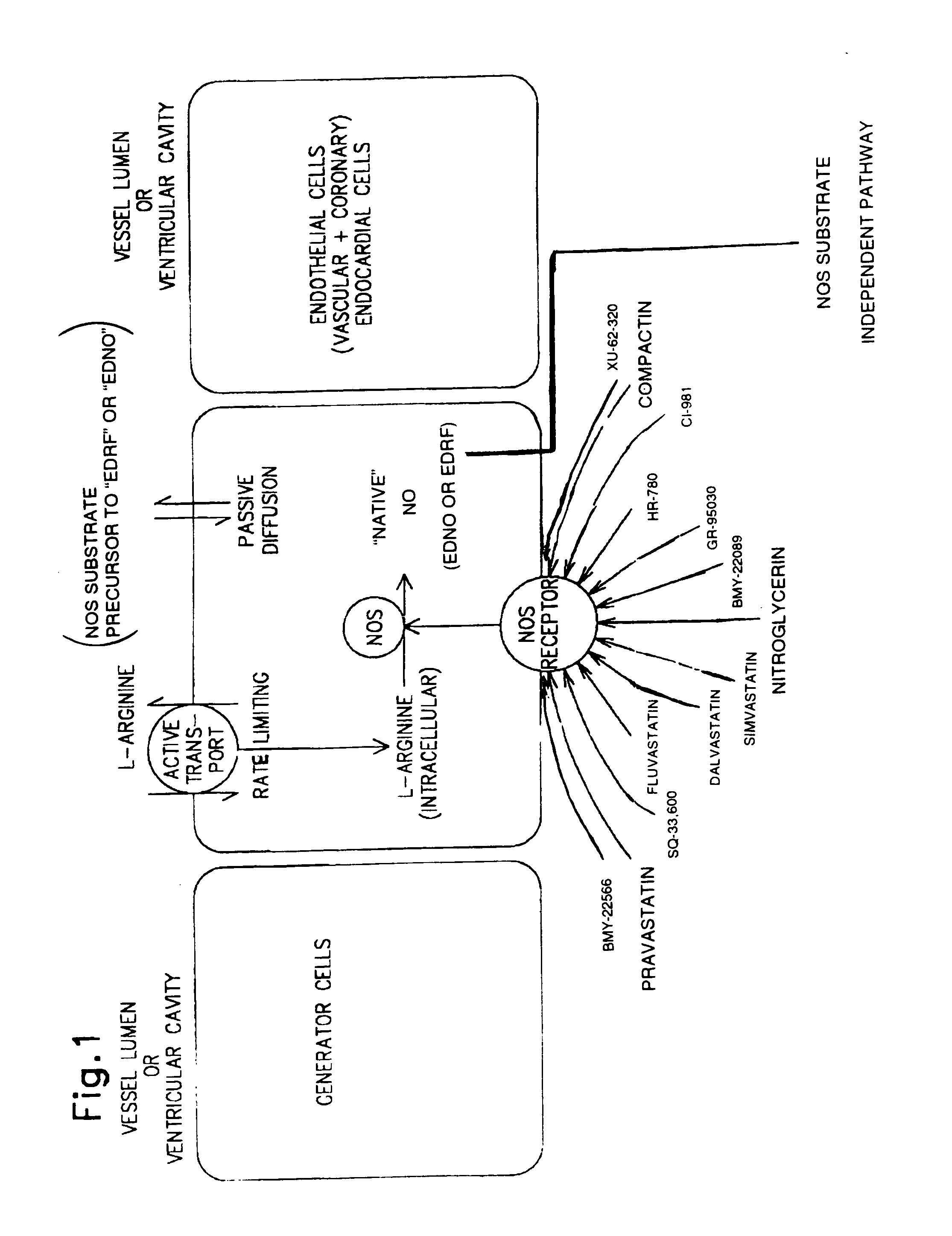
Method and formulation for treating vascular disease
a cardiocerebrorenovascular disease and formulation technology, applied in the field of treating cardiocerebrorenovascular disease, can solve the problems of no known research indicating the ability of hmg-coa reductase inhibitors to function, hypercholesterolemia is known to be a primary risk factor for death from coronary heart disease, etc., to achieve the effect of reducing endpoints, preventing or treating cardiovascular disease, and maximizing “native” no production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
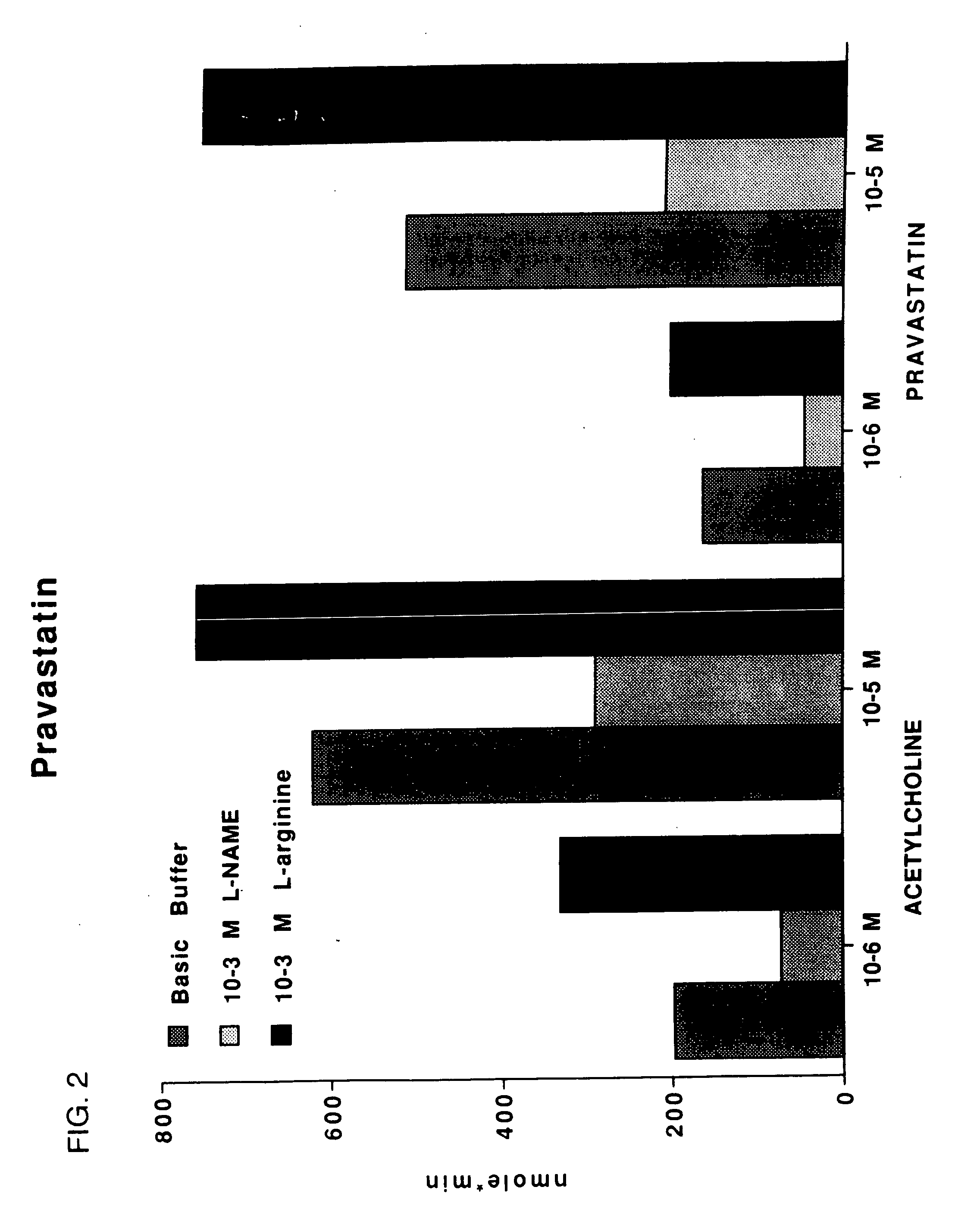
[0044] The direct effects of acteylcholine and pravastatin on NO production in bovine aortic endothelial cells (BAEC) was determined using a highly sensitive photometric assay for conversion of oxyhemoglobin to methemoglobin. NO oxidize; oxyhemoglobin (HbO2) to methemoglobin (metHb) in the following reaction HbO2+NO−metHb+NO3. The amount of NO produced by endothelial cells was quantified by measuring the change in absorbance as HbO2 oxidizes to metHb. Oxyhemoglobin has a absorbance peak at 415 nm, while metHb has a 406 nm absorbance peak. By subtracting the absorbance of metHb from HbO2, the concentration of NO can be assessed. The general method was patterned after that of Feelisch et al., (Biochem. and Biophy. Res. Comm. 1991; 180, Nc I:286-293).
[0045] For this assay, endothelial cells were isolated from bovine aortas. BAECs were grown to confluency in 150 mm plates (Corning) using Medium 199 supplemented with penicillin G (100 mL−1), streptomycin (100 mg mL−1), glutamine (100 mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| blood pressure | aaaaa | aaaaa |
| blood pressure | aaaaa | aaaaa |
| Total Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com