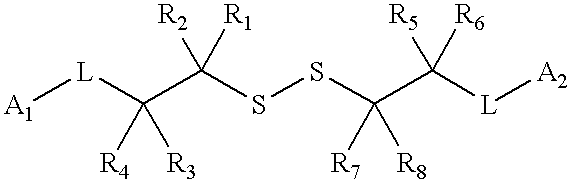
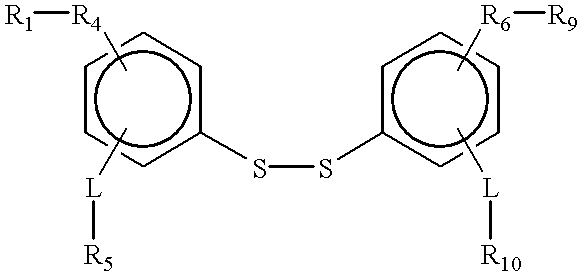
Compound containing a labile disulfide bond
a technology of labile disulfide and compound, which is applied in the field of compound containing labile disulfide bonds, can solve the problems of slow rate at which these bonds are broken under physiological conditions, and achieve the effect of lowering the pka of the constituent thiols
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Cysteine-terminal Tat Peptide (Tat-Cys).
Peptide syntheses were performed using standard solid phase peptide techniques using FMOC chemistry. A cysteine was added to the amino terminus of Tat to allow for conjugation through the thiol group to make the peptide Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Cys (Tat-Cys).
example 2
Synthesis of Noncleavably Linked (Irreversible Covalent) Tat-Cys and Fluorescein through a Thioether Bond.
To a solution of succinimidyl-4-(N-maleimidomethyl) cyclohexane-carboxylate (SMCC from Pierce) 1.0 mg in 0.1 mL dimethylformamide was added 1.2 mg (1 eq) of 4′-(aminomethyl)fluorescein. After two hours, this solution was added to a 1 mL aqueous solution of 8.4 mg Tat-Cys (1 eq). The solution was buffered to pH 8 by the addition of potassium carbonate. This solution was used for transport studies without further purification.
example 3
Synthesis of Unactivated (Non-labile) Disulfide Linked Lissamine Dimer (Dilissamine Cystamine)
To a solution of cystamine dihydrochloride (10 mg) in water (1 mL) was added diisopropylethylamine (15 μL, 2 eq). To this was added lissamine chloride (Rhodamine B sulfonyl chloride, Molecular Probes) 77 mg (3 eq) in 5 mL of methanol. The solution was stirred for 1 hour and then chromatographed by reverse-phase HPLC using an Aquasil C-18 column using a gradient from 100% 0.1% trifluoroacetic acid in water to 100% 0.1% triflouroacetic acid in acetonitrile. The fraction containing the product was determined by mass spectroscopy. The molecular weight of compound is 1234, which was detected in positive ion mode. The concentration of the product-containing fraction was determined by the absorbance of the solution at 588 nm (ε=88,000 M−1 cm−1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| half life | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com