Flexible vascular inducing implants
a vascular induction and implant technology, applied in the field of flexible vascular induction implants, can solve the problems of ischemic tissue infarction, permanent non-function, pain in the affected area, etc., and achieve the effects of increasing blood flow, high degree of flexibility, and maximizing blood exchang
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
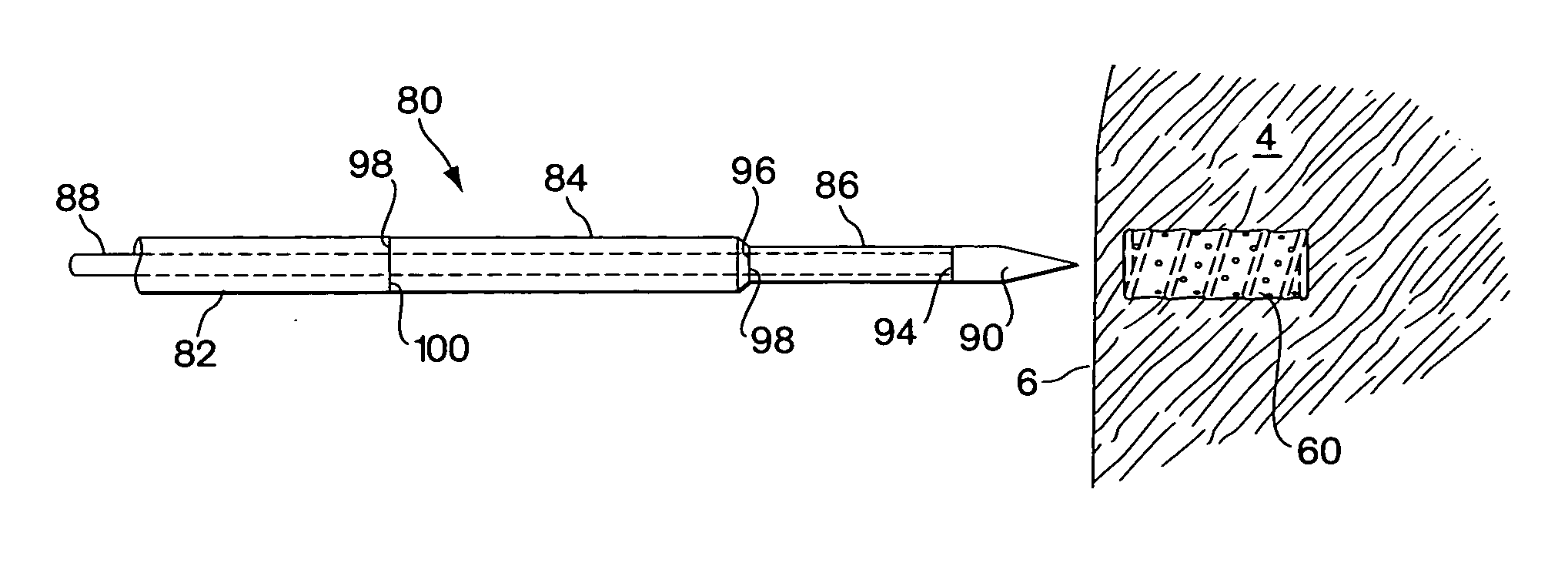
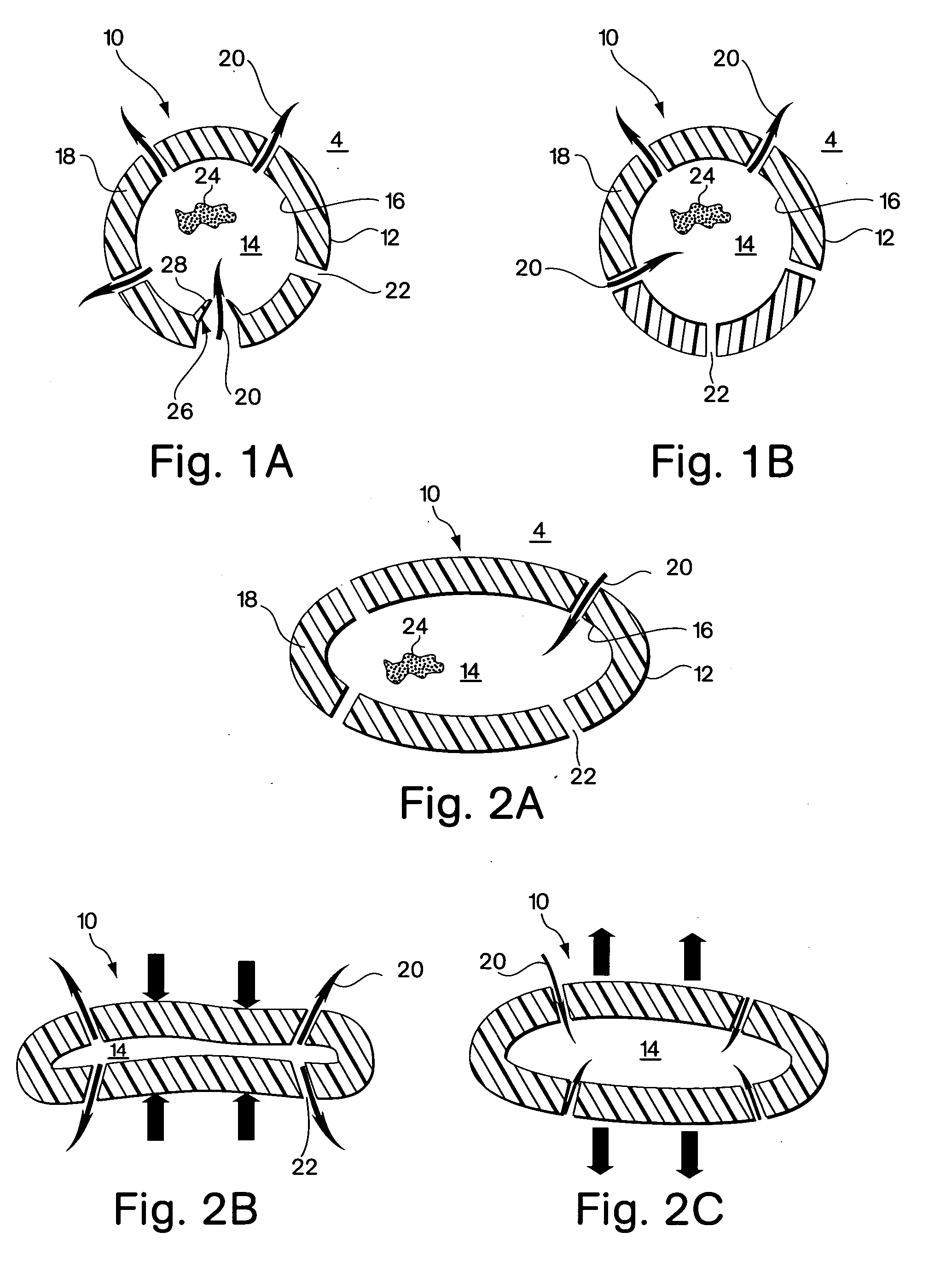
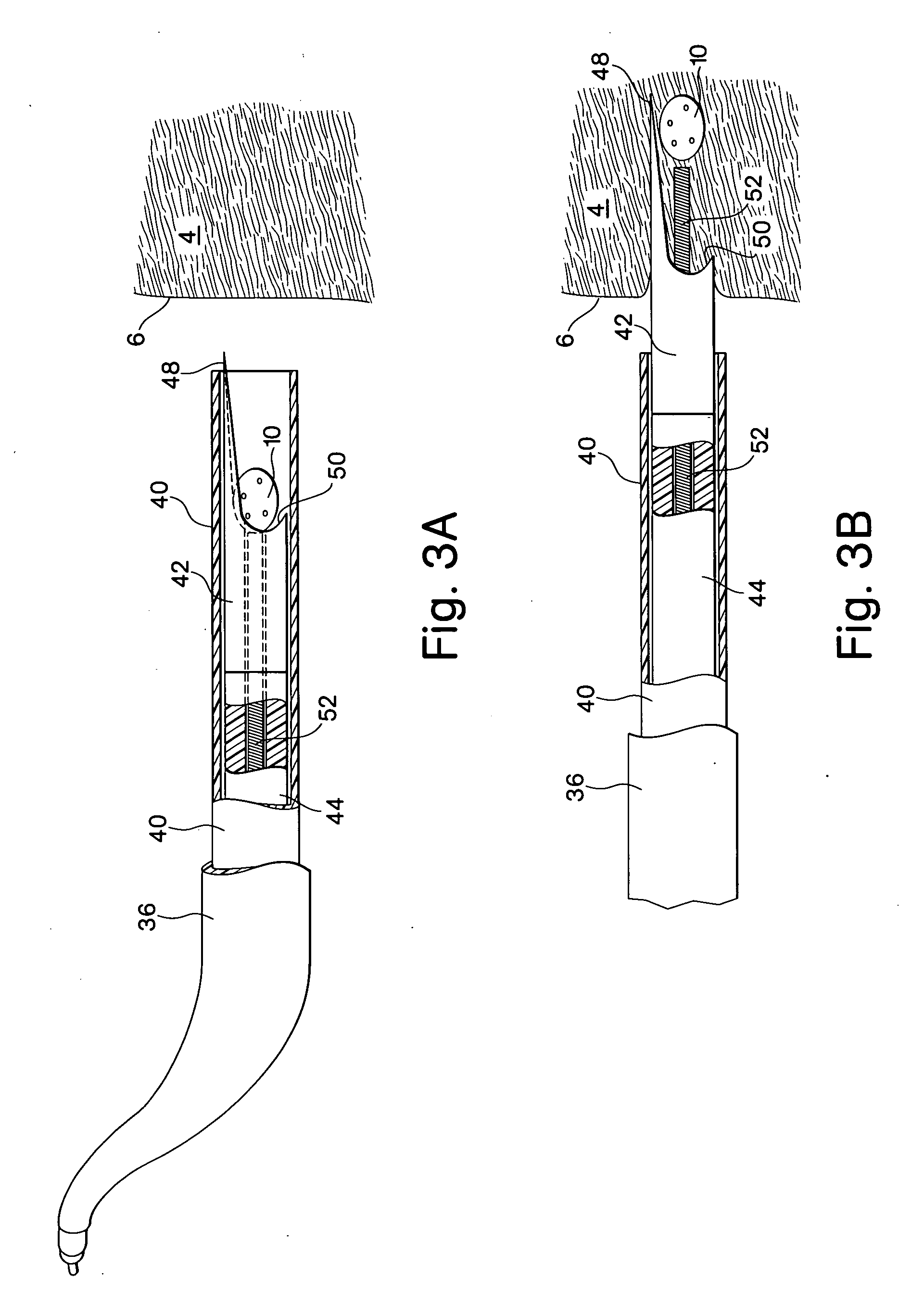
[0055]FIGS. 1A and 1B show one embodiment of the implant device comprising a capsule 10. The capsule embodiment has an exterior surface 12, a volume defining interior 14 with an inside surface 16. The wall 18 of the capsule may be somewhat flexible to permit flexure with the movement and compressive forces of the surrounding tissue 4 into which it is implanted. However, the wall should be fabricated to provide sufficient structural support to resist complete collapse of the capsule when it flexes.
[0056] Blood flow, represented by arrows 20, is intended to enter and exit the implant as part of the function of the device. As shown in FIG. 1B, blood 20 from the surrounding tissue 4 enters the interior 14 of the implant 10 through an opening 22. There may be several additional openings 22 to increase the amount of blood that can be exchanged through the device. Movement of the surrounding myocardial tissue 4 with the pumping of the heart flexes the capsule 10 and promotes blood interch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com