Gastrin compositions and formulations, and methods of use and preparation
a technology of compositions and formulations, applied in the field of gastrin compositions and formulations, and methods of use and preparation, can solve the problems of short plasma half-life, lower drug concentration than is required to be effective, and insufficient clearance of a therapeutic agent, so as to achieve the effect of high therapeutic efficacy and short plasma half-li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0100] Pharmacokinetic Example of Unmodified Gastrin Following Administration by Intravenous Injection to Male Cynomolgus Monkeys
[0101] This example was conducted to assess the pharmacokinetic (PK) profile of unmodified gastrin (referred to herein as compound B; see Table 3) following administration by a single intravenous injection to male cynomolgus monkeys. The 17 amino acid gastrin analog used has a single amino acid change at position 15, where methionine has been substituted by leucine. While compound B thus is not identical to naturally occurring gastrin, all available evidence indicates that it is functionally equivalent to gastrin.
[0102] Terms associated with PK analysis terms are defined as follows:
[0103] C.sub.max--The maximum observed plasma concentration
[0104] t.sub.max.--The time to maximum concentration
[0105] AUC--Area under the curve, a measure of total exposure to a drug over a period of time
[0106] Plasmat.sub.1 / 2--A measure of how long a drug stays in the blood. th...
example 2
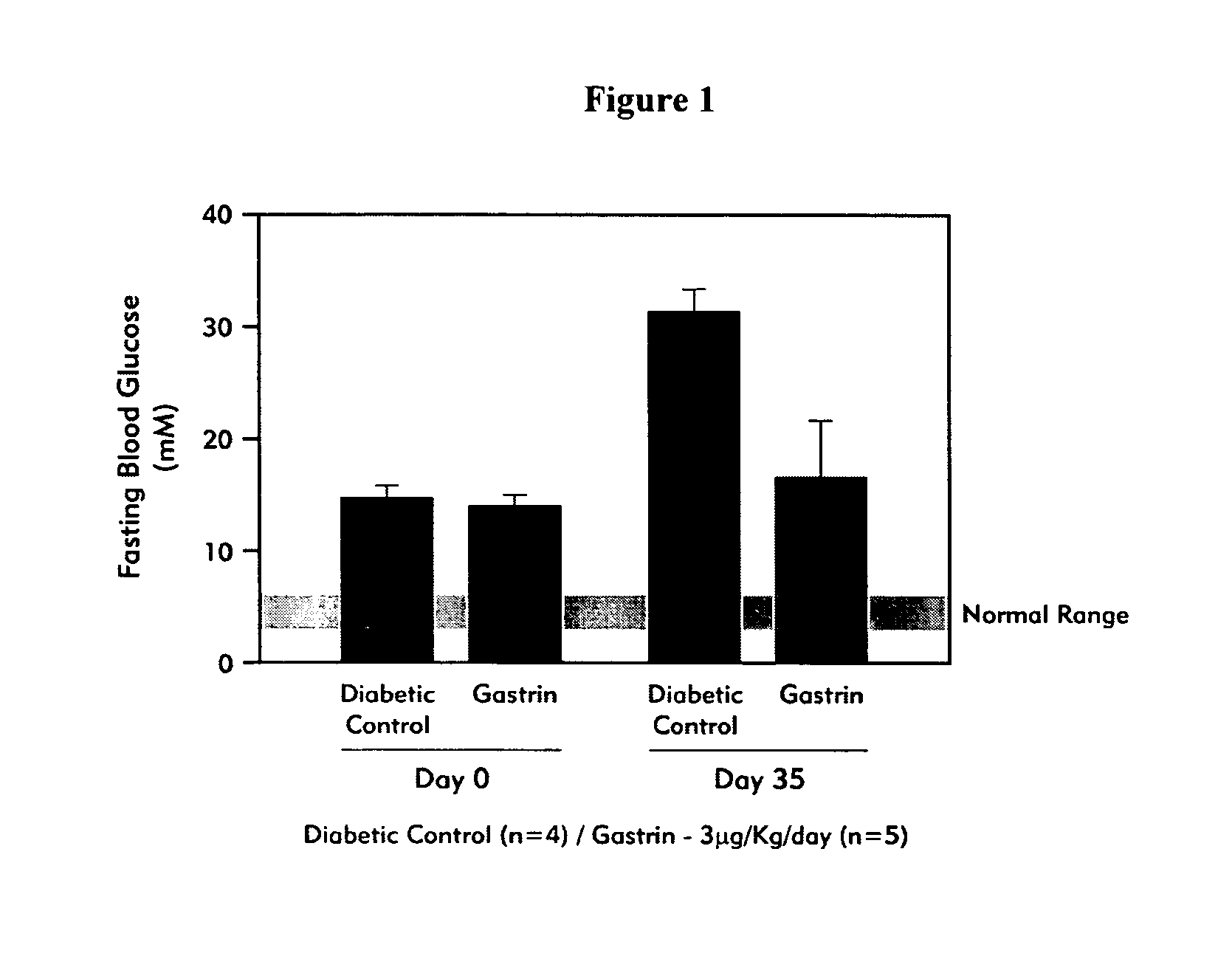
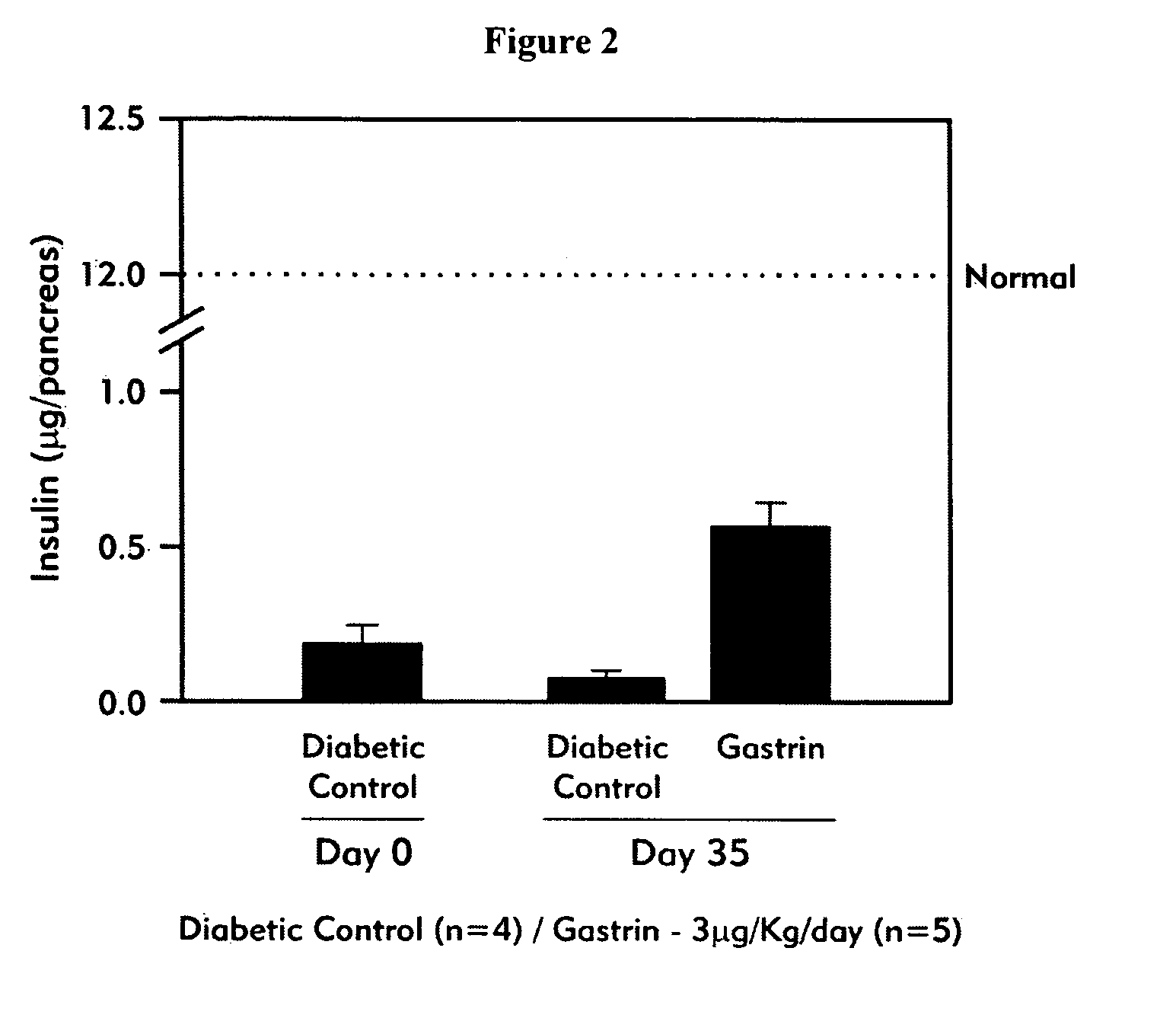
[0117] Effect of Unmodified Gastrin on Fasting Blood Glucose Levels and Pancreatic Insulin Content in NOD Mice with Recent Onset Diabetes
[0118] Non-obese diabetic (NOD) female mice were monitored for diabetes development as determined by a fasting blood glucose (FBG) level of >6.6 mmol / l. After diabetes onset, mice were treated with (i) vehicle (n=4); or, (ii) gastrin (compound B as listed in Table 3) in the amount of 3 .mu.g / kg / day, given i.p. once daily (n=5) for 14 days. Mice did not receive insulin-replacement treatment. Fasting blood glucose levels and pancreatic insulin content were assessed for the two treatment groups at both day 0 and day 35 (21 days after cessation of treatment).
[0119] FIG. 1 shows that in the vehicle-treated control animals, fasting blood glucose levels (FBG) were doubled after 35 days. In contrast, treatment with gastrin prevented some of the increase in glucose levels from rising in the diabetic NOD mice, however, the FBG levels remained significantly h...
example 3
[0121] Peptide Synthesis of Gastrin Peptides
[0122] Gastrin peptides may be readily synthesized by anyone with ordinary skills in the art, using standard techniques for solid phase peptide synthesis, for example as described by Steward, J. M. and Young, J. D. (1984) in "Solid Phase Peptide Synthesis", 2.sup.nd ed., Pierce Chemical Company. Purification of gastrin peptides may be performed using standard techniques, for example using reverse phase HPLC with a volatile binary gradient system consisting of 0.1% TFA in H.sub.2O and 0.1% TFA in acetonitrile. Monitoring elution by UV absorbance allows for collection of purified peptide, which is then lyophilized to dryness and subsequently dissolved for administration and testing, or for further conjugation reactions where required.
[0123] Gastrin synthetic peptides may be synthesized containing any consecutive portion of residues 1-28 in addition to residues 29-34 of SEQ ID NO: 1 or 2 or containing any consecutive portion of residues 1-11 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com