Compositions and methods for the treatment of primary and metastatic neoplastic diseases using arsenic compounds
a neoplastic disease and composition technology, applied in the direction of phosphorous compound active ingredients, drug compositions, amide active ingredients, etc., can solve the problems of dysplastic cells often having abnormally large nuclei, pleomorphism, and cancer incidence is continuing to climb, so as to improve the quality of life of patients and limit blood flow. , the effect of improving the quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
working examples
6. WORKING EXAMPLES
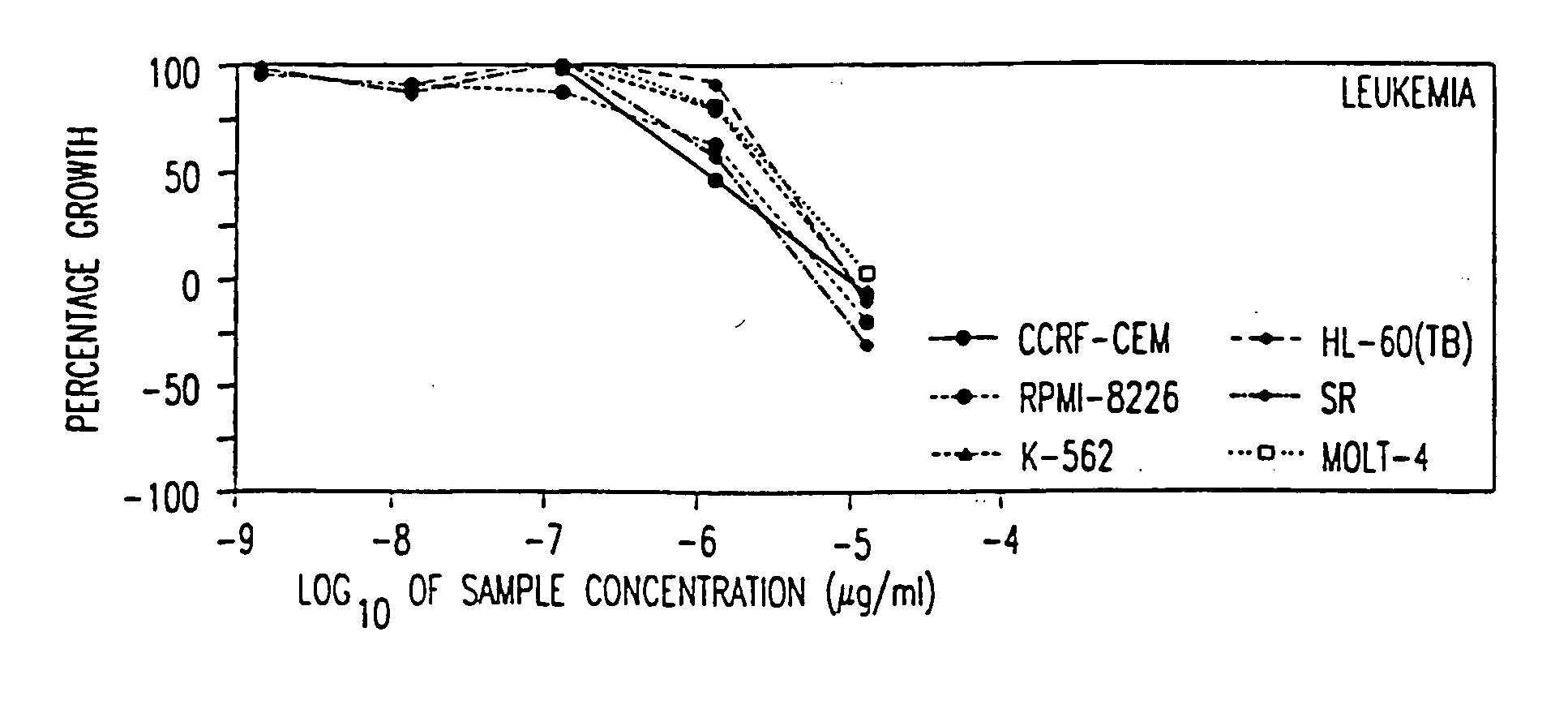
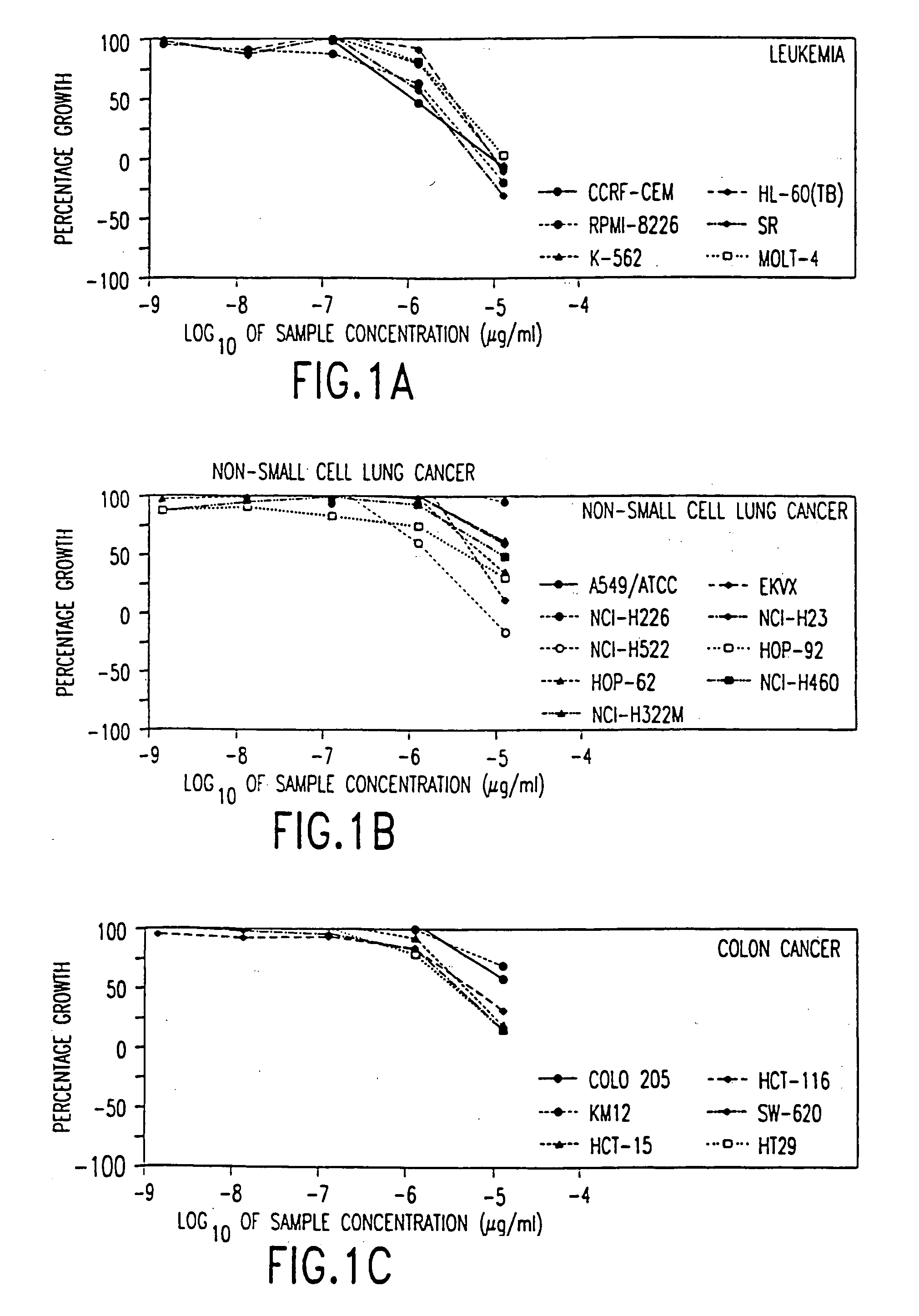
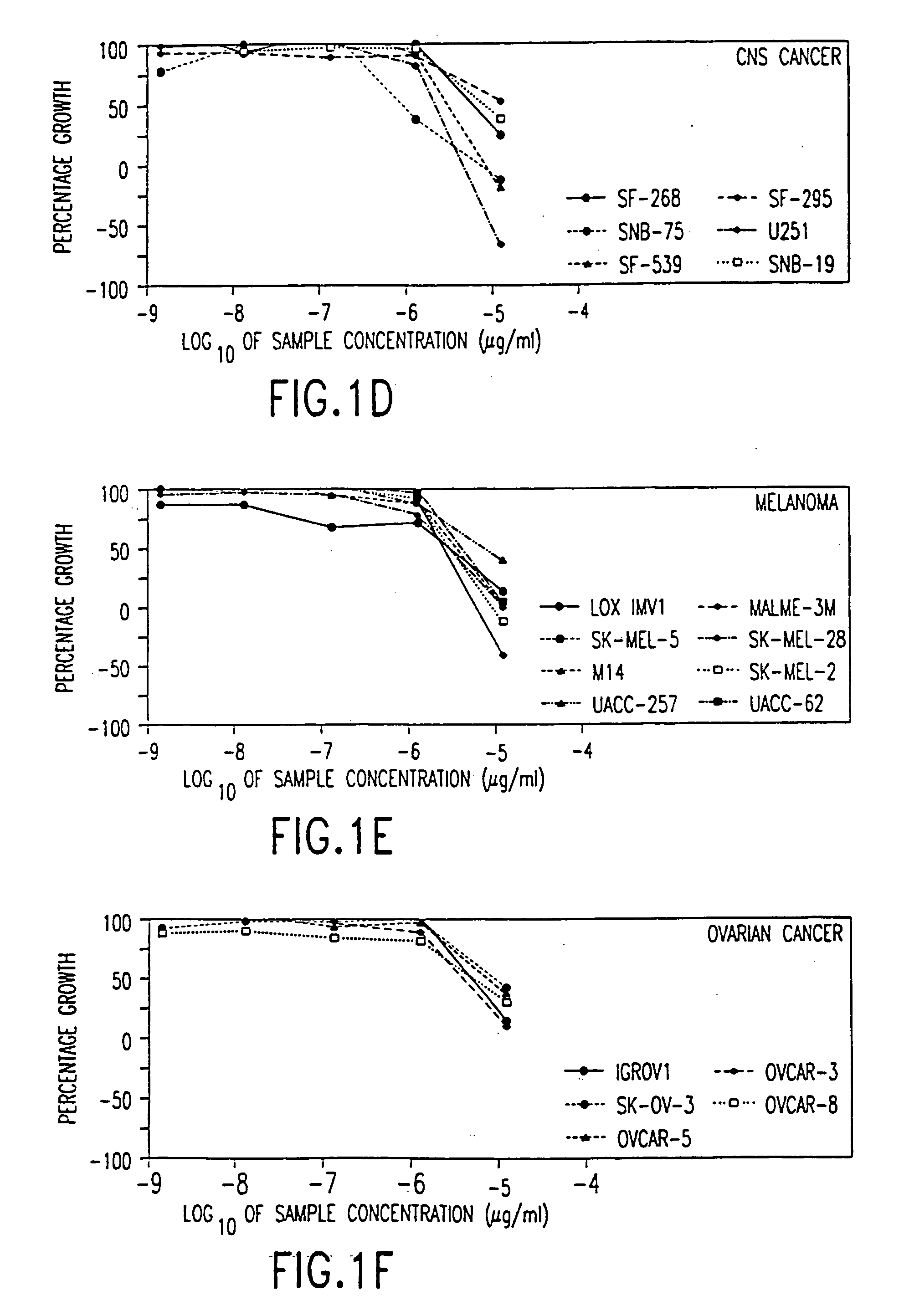
[0133] The following subsections describe the testing of a pharmaceutical composition comprising arsenic trioxide in vitro using a panel of cancer cell lines employed by the National Cancer Institute (NCI). The results demonstrate that arsenic trioxide is effective in inhibiting the growth of a broad range of leukemic cells and cancer cells in vitro.
6.1. Methods and Materials
[0134] Arsenic trioxide (1 mg / ml, 10 mg / ampoule, manufactured by Taylor Pharmaceuticals, Decatur., Ill.) was tested at five concentrations each at 10-fold dilutions, i.e., 10.sup.-5, 10.sup.-6, 10.sup.-7, 10.sup.-8, and 10.sup.-9 .mu.g / ml.
[0135] The in vitro tests were performed by incubating the test cells in the presence of the indicated concentration of arsenic trioxide under standard culture conditions for a designated period of time, which is followed by a sulforhodamine B (SRB) protein assay to estimate cell viability or growth. The cell lines are organized into subpanels according to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com