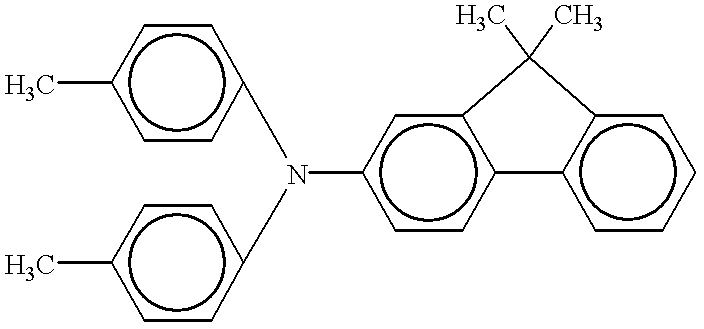
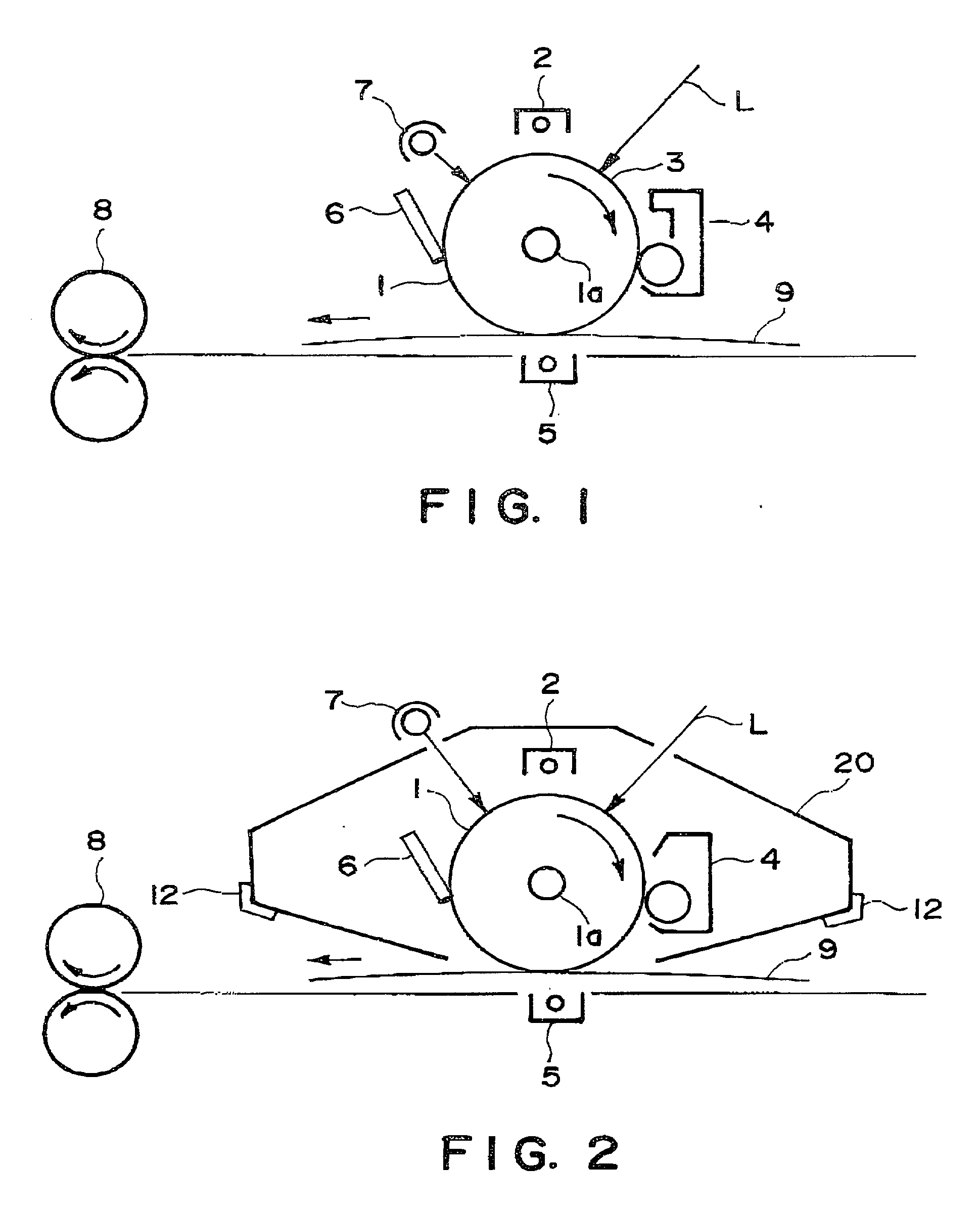
Phthalocyanine crystal, production process therefor, and electrophotographic photosensitive member, process cartridge and apparatus using the crystal
a technology of phthalocyanine crystal and production process, which is applied in the field of phthalocyanine crystal, can solve the problems of low conductivity, difficult commercialization of gallium phthalocyanine crystal, and liable to fluctuation of photosensitive members using gallium phthalocyanine crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
synthesis example 2
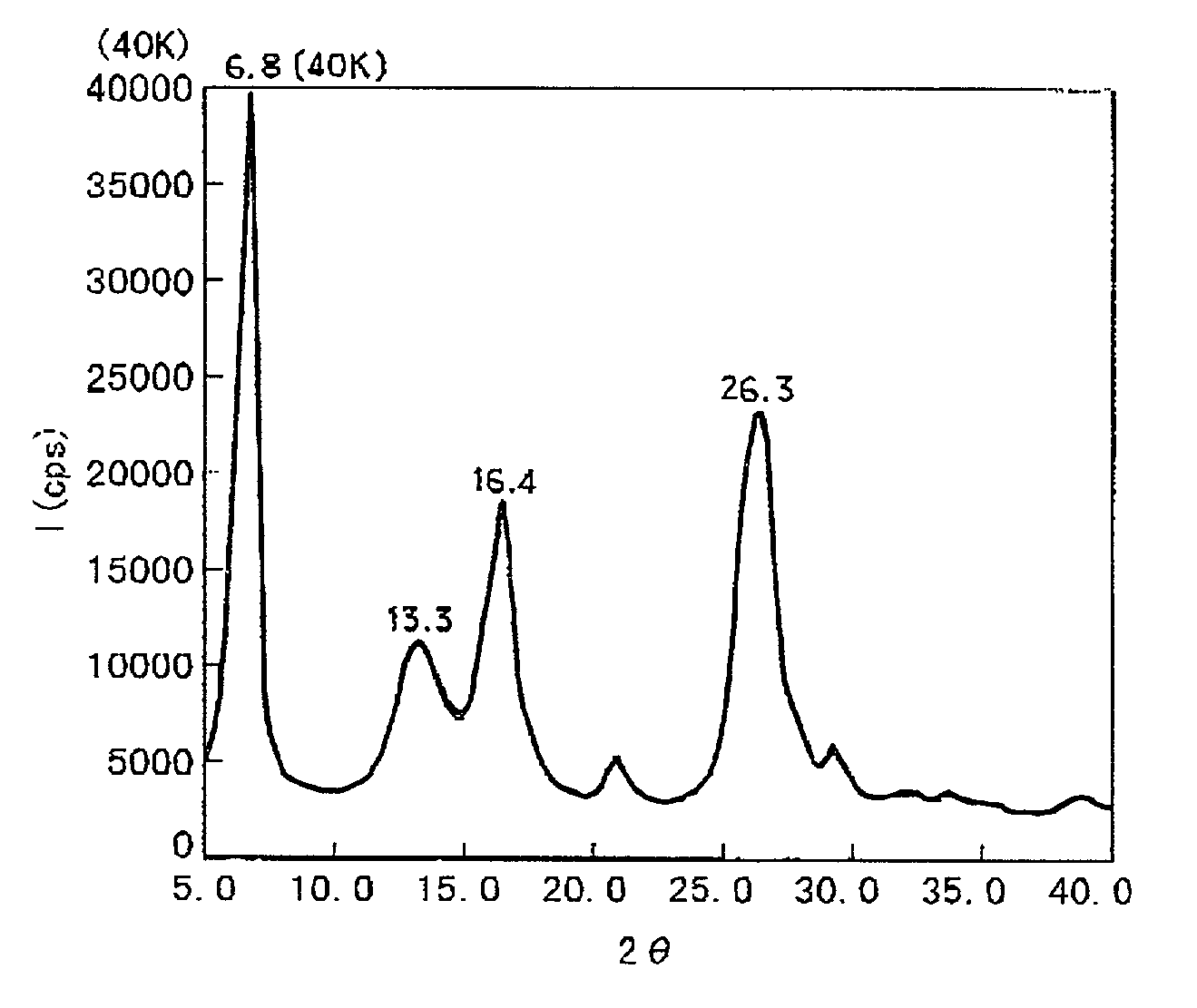
[0066] 15 parts of the chlorogallium phthalocyanine prepared in Synthesis Example 1 was dissolved in 300 parts of conc. sulfuric acid cooled at 15.degree. C., and the resultant solution was added dropwise into 2000 parts of iced water under stirring to cause re-precipitation, followed by filtration. The precipitate was washed in dispersion first within 2% ammonia water and then four times within deionized water, and then dried in vacuum at 40.degree. C. to obtain 13 parts of hydroxygallium phthalocyanine crystal. The hydroxygallium phthalocyanine crystal (represented by C.sub.32H.sub.17GaN.sub.8O) exhibited a powdery X-ray diffraction pattern as shown in FIG. 6 and the following results of elementary analysis.
2 Element Calculated (%) Measured (%) C 64.1 63..2 H 2.9 3.2 N 18.7 18.3 Cl 0.0 0.0
example 1
[0067] 15 parts of the chlorogallium phthalocyanine prepared in Synthesis Example 1 was dissolved in a mixture of 300 parts of conc. sulfuric acid and 1.5 parts (corresponding to 10% of the chlorogallium phthalocyanine) of .alpha.-chloronaphthalene (purity >85%, available from Tokyo Kasei Kogyo K.K.) cooled at 15.degree. C., and the resultant solution was added dropwise into 2000 parts of iced water under stirring to cause re-precipitation, followed by filtration. The precipitate was washed in dispersion first within 2% ammonia water and then four times within deionized water, and then dried in vacuum at 40.degree. C. to obtain 13 parts of hydroxygallium phthalocyanine crystal, which exhibited a powdery X-ray diffraction pattern as shown in FIG. 7.
example 2
[0068] 15 parts of the chlorogallium phthalocyanine prepared in Synthesis Example 1 was dissolved in 300 parts of conc. sulfuric acid cooled at 15.degree. C., and the resultant solution was added dropwise into a mixture of 2000 parts of iced water and 15 parts (corresponding to 100% of the chlorogallium phthalocyanine) under stirring to cause re-precipitation, followed by filtration. The precipitate was washed in dispersion first within 2% ammonia water and then four times within deionized water, and then dried in vacuum at 40.degree. C. to obtain 13 parts of hydroxygallium phthalocyanine crystal, which exhibited a powdery X-ray diffraction pattern as shown in FIG. 8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com