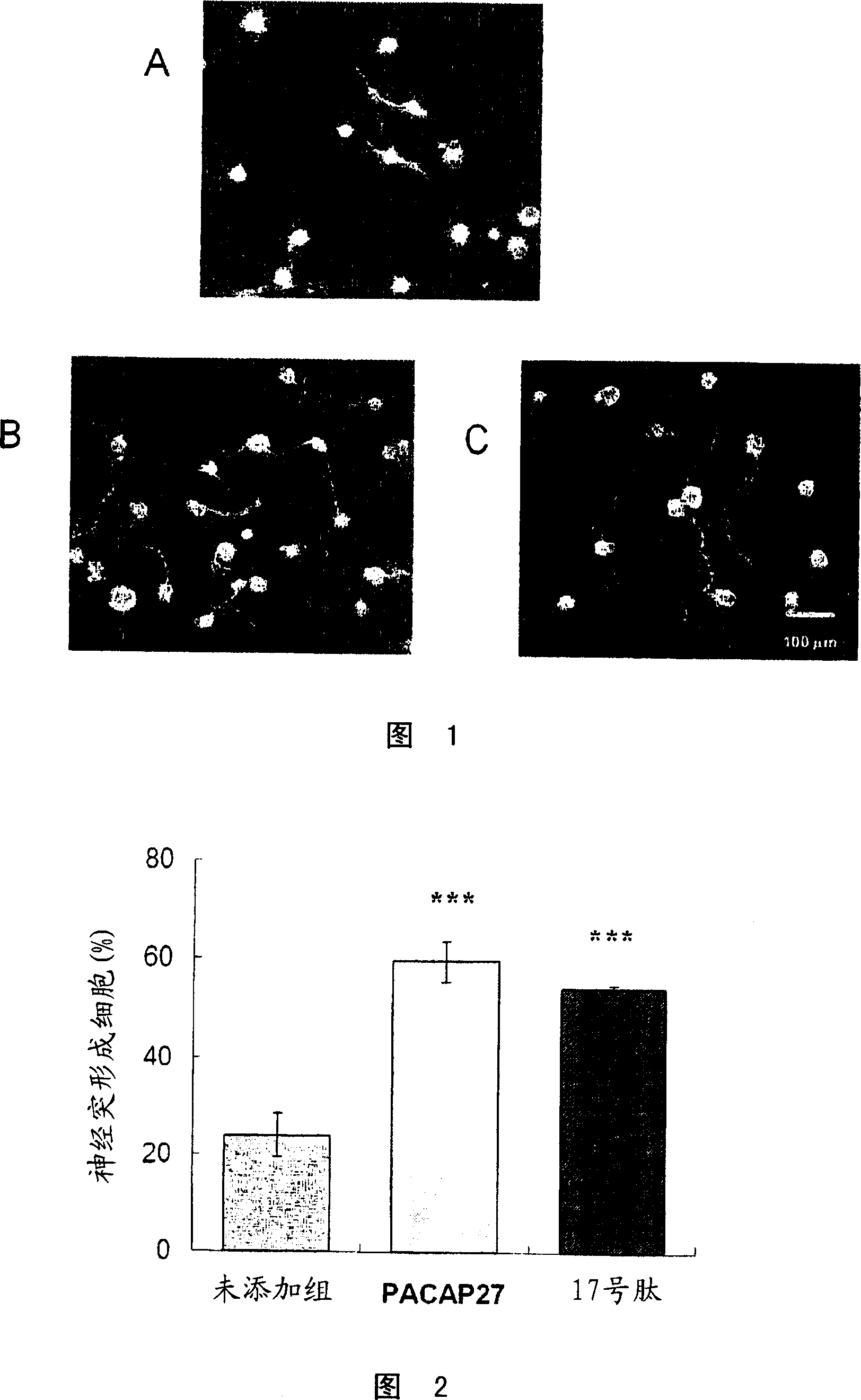
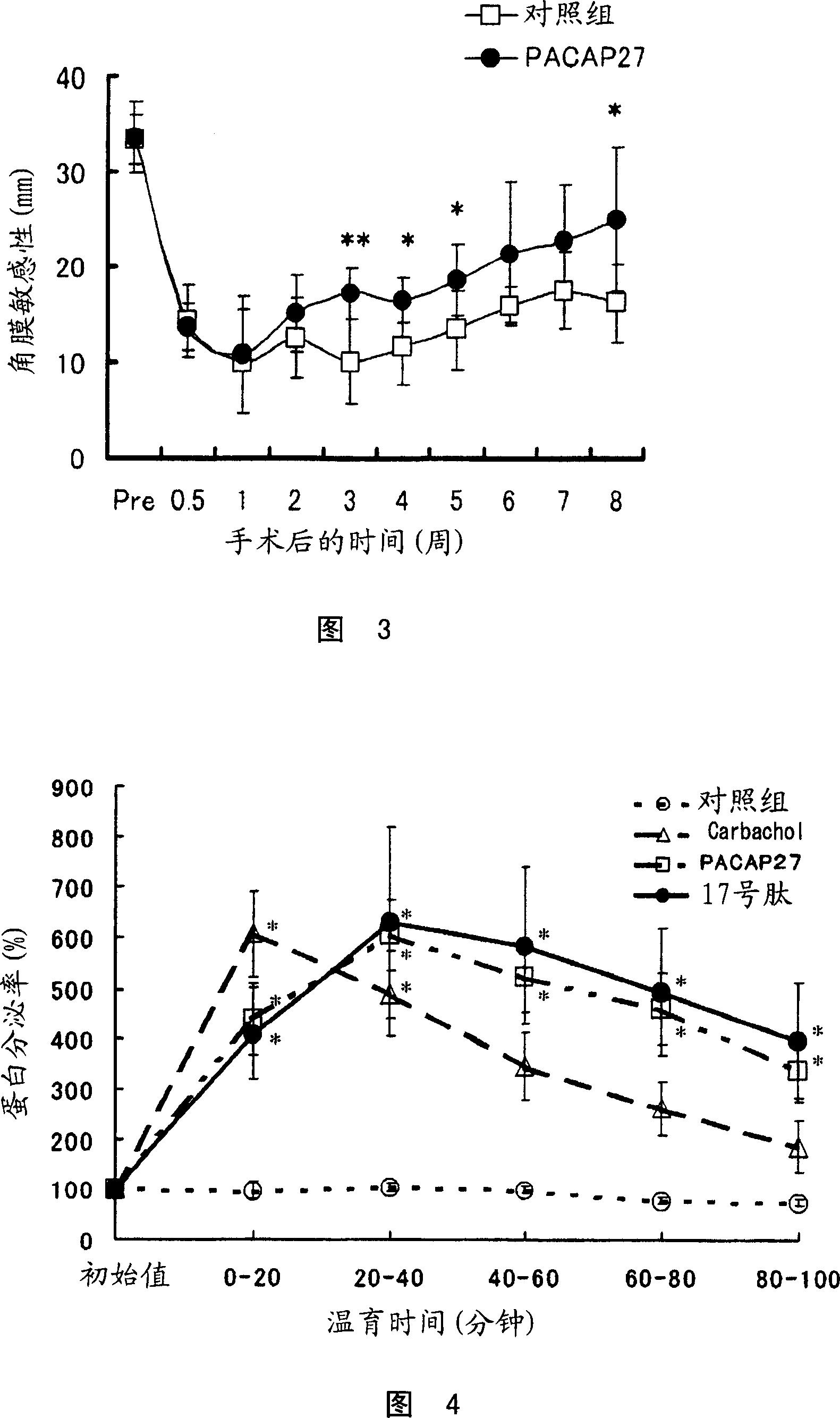
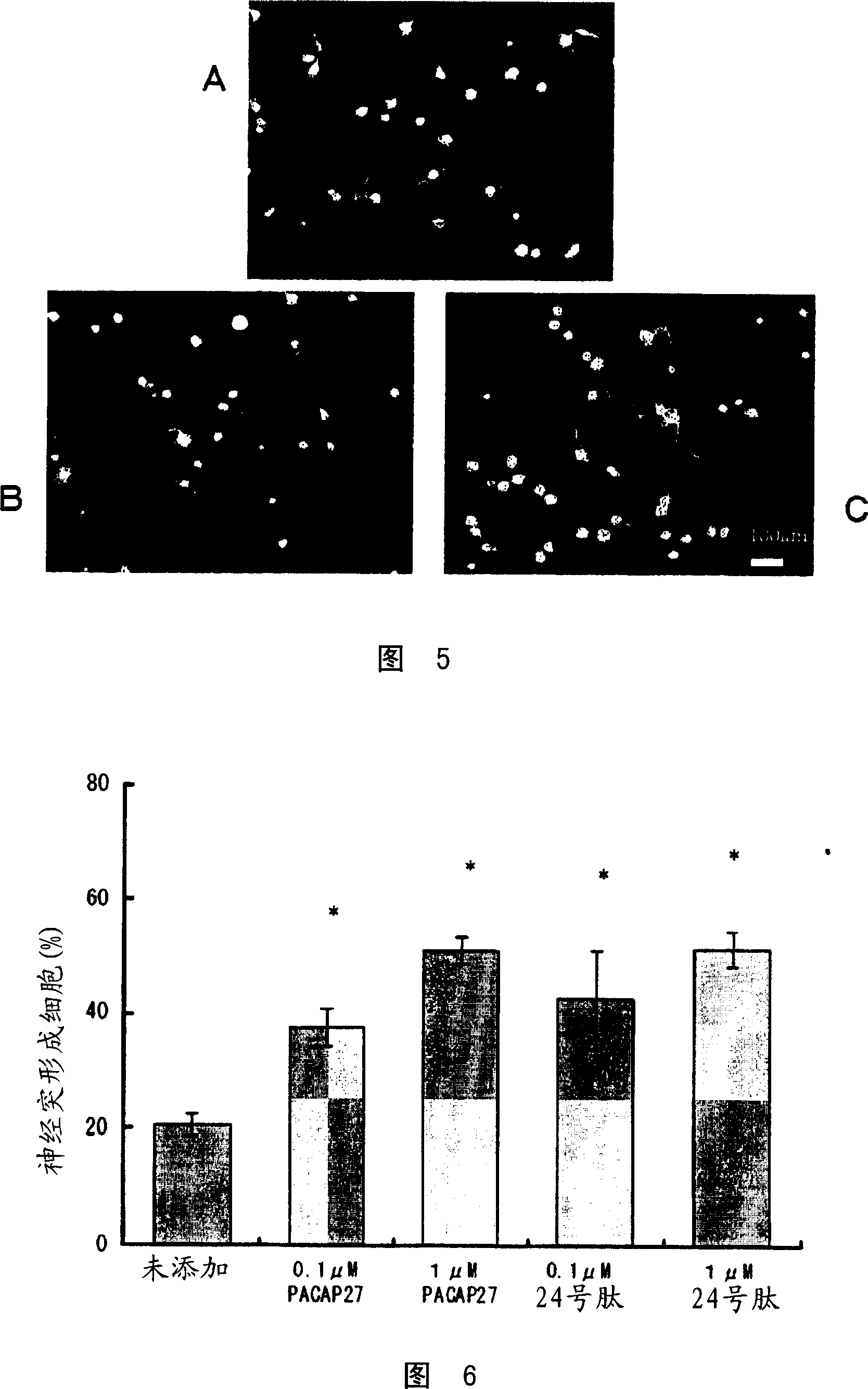
Corneal neuritogenesis promoter containing PACAP and its derivative
A technology of derivatives and neurites, applied in the field of reagents for improving corneal sensitivity and therapeutic agents for corneal epithelial damage, can solve the problems of no indication or indication, no indication or indication indication, etc., so as to improve corneal sensitivity reduction and dryness. Effects of eye symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] peptide synthesis
[0113] Peptide No. 3 having the amino acid sequence shown in SEQ ID No. 19 was prepared by a conventional method for solid-phase peptide synthesis.
[0114] The hydrochloride (polystyrene-1% divinylbenzene copolymer, 100-200 mesh) of MBHA resin is placed in artificial synthesis reactor (made by glass, 6.0cm (diameter) * 29.5cm (height)) , wash with methanol 2-3 times the volume of the resin while stirring, and then wash with diaminomethane (2-3 times the volume of the resin) while stirring to expand the resin. The resin was neutralized with 10% triethylamine in diaminomethane by using dicyclohexylcarbodiimide and N-hydroxybenzotriazole with approximately 2 equivalents of Boc-Arg (Tos )-OH for condensation. After the condensation reaction proceeded for about 2 hours (with stirring), the resin was washed with methanol and dichloromethane. After disappearance of the α-amino group was confirmed by the Kaiser test, the resin was treated with 50% triflu...
Embodiment 9
[0326] Facilitating effect on the treatment of rabbit corneal epithelial injury
[0327] 1. Animals used
[0328] Male Japanese white rabbits weighing 2.0-2.5 kg purchased from Kitayama Labes Co., Ltd. were used. Each animal was placed in a separate cage indoors, which maintained a temperature of 23 ± 3°C and a humidity of 55 ± 10%, and was illuminated for 12 hours a day (lighting started at 8:00 and light ended at 20:00), and the experiment was carried out after arrival. Rearing until the last day of testing. Animals were given 100 g of solid feed (Labo R Stock, Nosan Corporation) per day, with free access to tap water.
[0329] 2. Test substance
[0330] PACAP27 was used as test substance. The test substances were dissolved in the following vehicle solutions at a concentration of 10 μM or 100 μM to prepare eye drops. As a control, the following vehicle solutions without test substances were used.
[0331] The formulation of the carrier solution:
[0332] Sodium dihydr...
Embodiment 2
[0352] Examples of pharmaceutical formulations are described below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com