Imidazoline modified amino acid, and its synthesizing method and use for polypeptide marking
A technology for synthesizing polypeptides and imidazolines, applied in the chemical field, can solve problems such as limitations and complex products, and achieve good biocompatibility, good chemical stability, completely consistent physical and chemical properties and biological activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
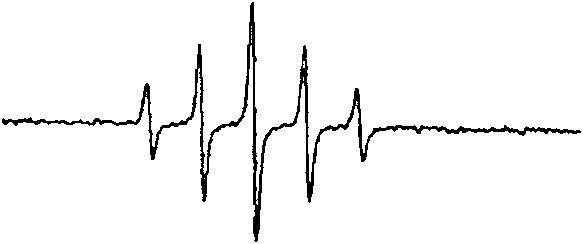
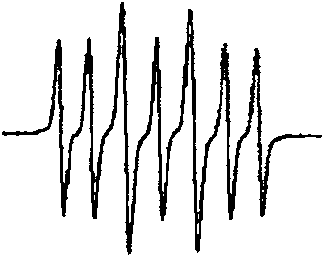
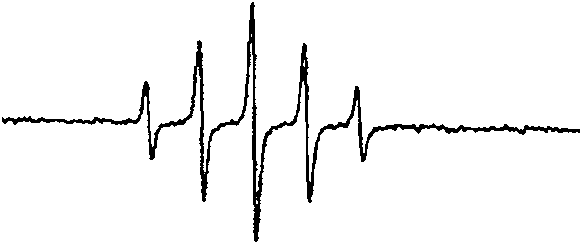
Image
Examples
preparation Embodiment 2
[0046] [Intermediate Preparation Example 2] Preparation of 2,3-dimethyl-2,3-dihydroxyaminobutane (compound 3)
[0047] From 10.0g (56.82mmol) 2,3-dimethyl-2,3-dinitropropane (2), 5.6g NH 4 Cl and 112ml ethanol solution (50% formed suspension was added 22.9g zinc powder under ice bath and stirring, lasted 3 hours. Then the reaction mixture was stirred at room temperature for 3 hours. The reaction mixture was filtered under reduced pressure, and the filter cake was washed with 50% ethanol aqueous solution Wash repeatedly. Combine the washing liquid and the filtrate, adjust the pH to 2 with concentrated hydrochloric acid, and concentrate under reduced pressure to a muddy state. The obtained muddy matter is mixed with an appropriate amount of K 2 CO 3 Mix well to pH 10, then place in a Soxhlet extractor with CHCl 3 Extract for 6 hours. The extract was concentrated under reduced pressure, and the residue was crystallized with petroleum ether to obtain 4.0 g (47.57%) of the title...
preparation Embodiment 3
[0048] [Intermediate Preparation Example 3] Preparation of 1,3-dihydroxy-2-(4'-hydroxyphenyl)-4,4,5,5-tetramethylimidazolidine (compound 4)
[0049] A solution composed of 990mg (8.107mmol) p-hydroxybenzaldehyde, 1.196g (8.078mmol) 2,3-dimethyl-2,3-dihydroxyaminobutane (3) and 4.8ml methanol was stirred at room temperature for 6 hours, TLC The raw material point disappeared, and the reaction mixture was filtered under reduced pressure to obtain 1.47 g (72%) of the title compound as colorless crystals, ESI-MS (m / e) = 236 [M] + ; f 0.64 (CHCl 3 / CH 3 OH, 10:1), mp: 168-169; IR (KBr, em -1 ): 3340, 1600, 1450; NMR (CDCl 3 )δ=1.14(s, 4-CH 3 ), 4.78 (s, CH), 7.31-7.51 (m, 5-ArH), 7.71 (2-OH). Elemental Analysis: C 13 h 20 N 2 o 2 Calculated for: C, 66.07; H, 8.53; N, 11.85.
preparation Embodiment 4
[0050] [Intermediate Preparation Example 4] Preparation of 1,3-dioxo-2-(4'-hydroxyphenyl)-4,4,5,5-tetramethyl-2-imidazoline (compound 5)
[0051] Add 8.14g PbO to the solution of 1.47g (5.83mmol) 1,3-dihydroxyl-2-(4'-hydroxyphenyl)-4,4,5,5-tetramethylimidazolidine and 81ml methanol 2 (34.40 mmol). After stirring at room temperature for 0.5 hr, TLC showed that the starting point disappeared. The reaction mixture was filtered, the filtrate was concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (petroleum ether / ethyl acetate, 2:1). The collected product-containing fractions were concentrated under reduced pressure to afford 1.06 g (73%) of the title compound as a blue solid. R f =0.54 (CHCl 3 / CH 3 OH, 20:1); mp 84-85°C; ESI-MS(m / e)=233[M] + ; IR(KBr, cm -1 ): 3325, 1610, 1500, 1450, 765; elemental analysis: C 13 h 17 N 2 o 2 Calculated for: C, 66.93; H, 7.34; N, 12.01.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com