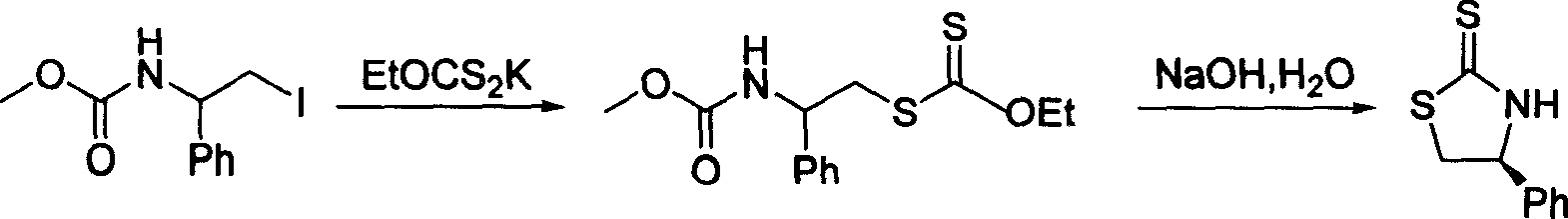
Process for synthesizing chiral thiazolidine-2-thioketone
A synthesis method and compound technology, which is applied in the field of synthesis of chiral thiazolidine-2-thione compounds, can solve the problems of long reaction time, expensive raw materials, difficult to avoid by-product oxazolidine-2-thione, etc. , to achieve the effect of simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
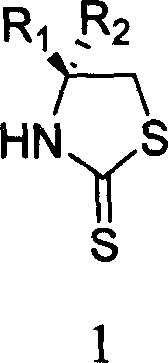
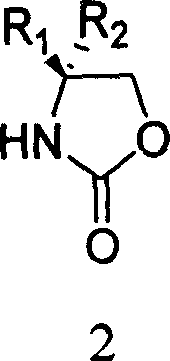
[0024] Add toluene (20mL) to the chiral oxazolidinone 1 (1.63g, 10mmol) under stirring at room temperature, after complete dissolution, add phosphorus pentasulfide (5.55g, 25mmol), heat and reflux for 3 hours, T.L.C shows that the reaction is complete, stop stirring, Let it stand for 10 minutes, and decant the supernatant liquid while it is hot. The remaining solid was cooled to room temperature, placed in an ice bath, and saturated NaHCO was slowly added under rapid stirring. 3 solution, the solid was completely dissolved, the solution was neutral, and then, ethyl acetate was added for extraction (20mL×3). The extracted organic phase was combined with the decanted clear liquid, and successively washed with saturated NaHCO 3 solution (40mL), water (40mL), washed with saturated brine (40mL), anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure at room temperature to obtain (R)-4-phenyl-thiazolidine-2-thione 2 (white solid, 1.72 g, y...
Embodiment 2
[0026]
[0027] Toluene (20mL) was added to the chiral oxazolidinone 3 (1.77g, 10mmol) under stirring at room temperature. After complete dissolution, phosphorus pentasulfide (5.55g, 25mmol) was added, and heated to reflux for 3 hours. T.L.C showed that the reaction was complete, and the stirring was stopped. Let it stand for 10 minutes, and decant the supernatant liquid while it is hot. The remaining solid was cooled to room temperature, placed in an ice bath, and saturated NaHCO was slowly added under rapid stirring. 3 solution, the solid was completely dissolved, the solution was neutral, and then, ethyl acetate was added for extraction (20mL×3). The extracted organic phase was combined with the decanted clear liquid, and successively washed with saturated NaHCO 3 solution (40mL), water (40mL), washed with saturated brine (40mL), anhydrous Na 2 SO 4 Drying and concentration to remove the solvent gave (S)-4-benzyl-thiazolidine-2-thione 4 (light yellow solid, 1.80 g, yi...
Embodiment 3
[0029]
[0030] Add toluene (20mL) to the chiral oxazolidinone 5 (1.29g, 10mmol) under stirring at room temperature. After complete dissolution, add phosphorus pentasulfide (5.55g, 25mmol), heat and reflux for 3 hours, T.L.C shows that the reaction is complete, stop stirring, Let stand for 10 minutes, and decant the supernatant liquid while hot. The remaining solid was cooled to room temperature, placed in an ice bath, and saturated NaHCO was slowly added under rapid stirring. 3 solution, the solid was completely dissolved, the solution was neutral, and then, ethyl acetate was added for extraction (20mL×3). The extracted organic phase was combined with the decanted clear liquid, and successively washed with saturated NaHCO 3 solution (40mL), water (40mL), washed with saturated brine (40mL), anhydrous Na 2 SO 4 After drying and concentration to remove the solvent, (S)-4-isopropyl-thiazolidine-2-thione 6 was obtained (yellow oil, 1.30 g, yield 81%). [α] D 19 -35.72° (c0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com