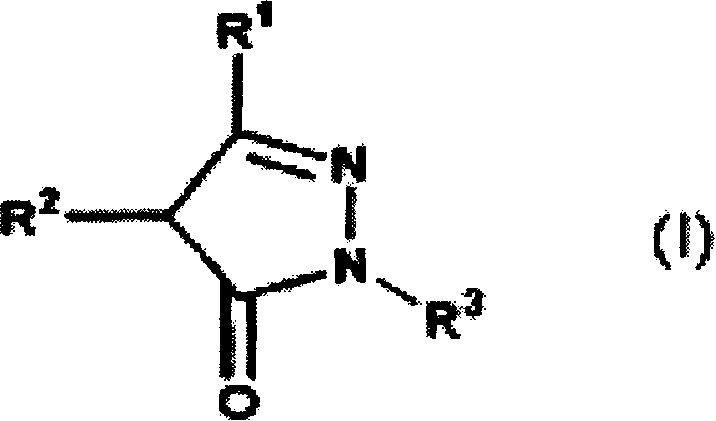
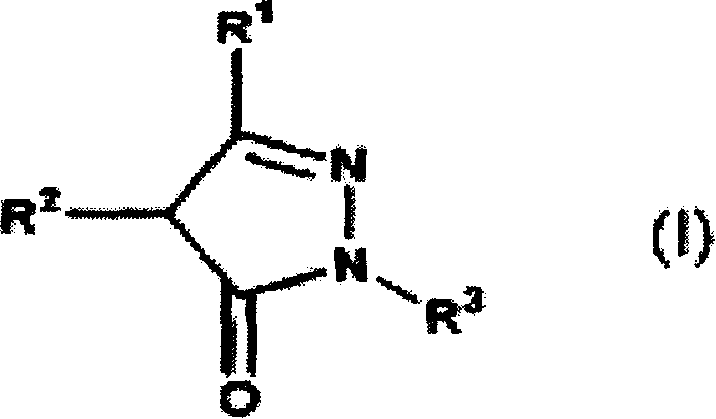
Novel therapeutic agent for amyotrophic lateral sclerosis(ALS) or disease attributable to ALS
A technology for lateral sclerosis and amyotrophy, which is applied in the fields of muscular system diseases, neuromuscular system diseases, drug combinations, etc., and can solve problems such as difficult to predict drug delivery methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Example 1 According to ALSFRS-R (Revised ALS Functional Rating Scale, reference: cranial nerve, 53 (4): 346-355, 2001), the effectiveness evaluation after half a year of administration.
[0171] (30mg group)
[0172] For 5 ALS patients, 1 ampoule of "Radicut 30 mg injection" (containing edaravone 30 mg, manufactured and sold by Mitsubishi Pharmaceutical Co., Ltd.) was intravenously administered once a day for 30 minutes each time for 14 consecutive days. Days (Phase 1 administration). After the end of the first period, a 2-week observation (drug withdrawal) period was set, and then the same intravenous administration (administration in the second period) was performed for 10 days (no administration on Saturdays, Sundays, and holidays). Afterwards, repeat the same measures as in the second phase of administration 4 times (the third to sixth phases of administration).
[0173] (60mg group)
[0174] To 14 ALS patients, 2 ampoules of the above-mentioned "Radicut 30 mg in...
Embodiment 2
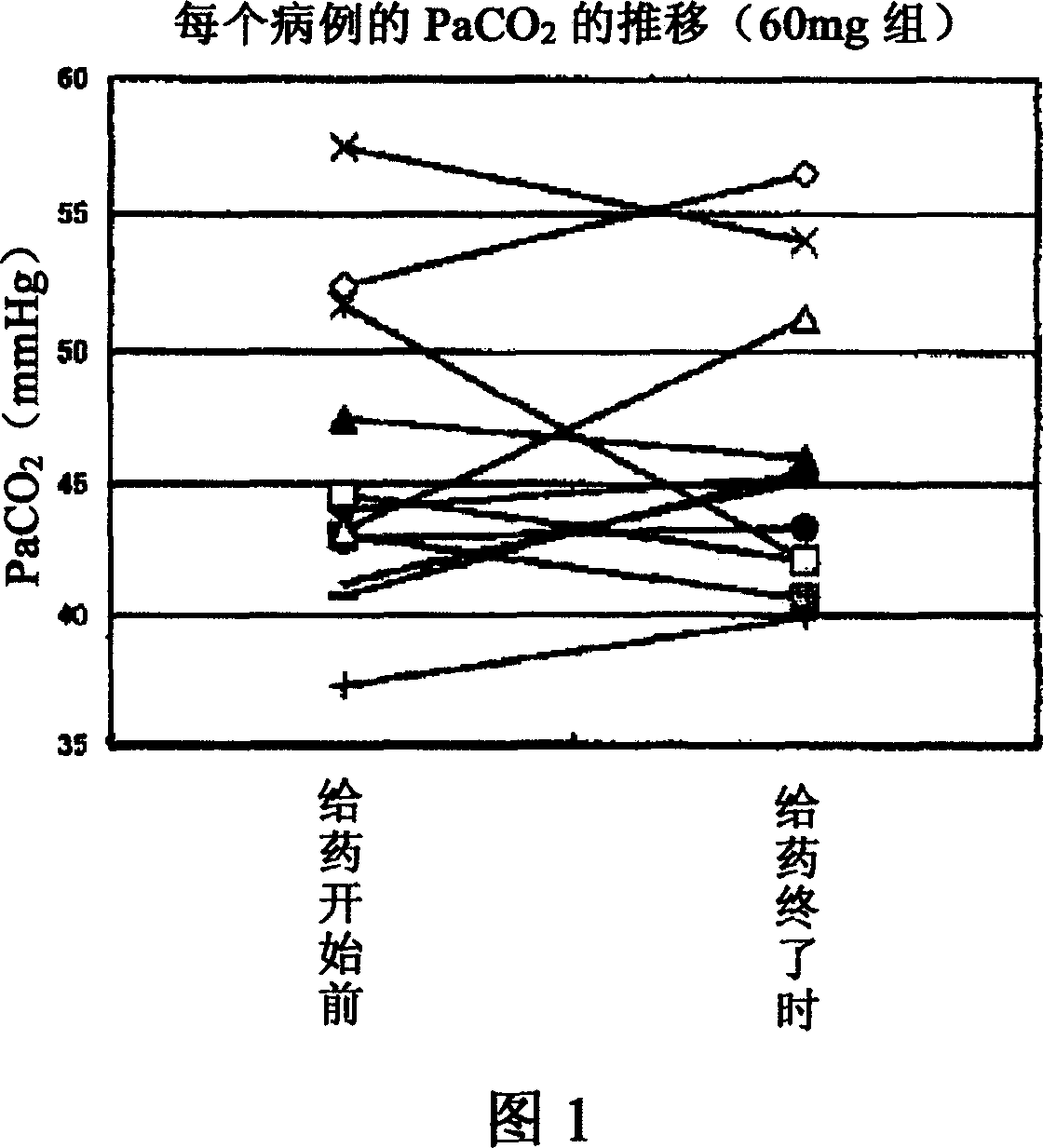
[0204] Example 2 by %FVC and PaCO 2 Effectiveness evaluation after half a year of administration
[0205] The so-called "%FVC" (percent-predicted forced vital capacity) is the forced vital capacity expressed in %, and is an index commonly used as an objective method of evaluating respiratory function of ALS patients (ALS Treatment Guidelines 2002). In addition, according to The BDNF Study Group (Phase III), Neurology, 52, 1427 (1999), the %FVC decrease rate of ALS patients was 13.8% (placebo group) in 6 months.
[0206] (30mg group)
[0207] To 4 ALS patients, 1 ampoule of the aforementioned "Radicut 30 mg injection" was intravenously administered once a day for 30 minutes each time for 14 consecutive days (the first period of administration). After the end of the first period, a 2-week observation (drug withdrawal) period was set, and then the same intravenous administration (administration in the second period) was performed for 10 days (no administration on Saturdays, Sun...
Embodiment 3
[0214] Example 3 Safety evaluation after administration for half a year
[0215] The clinical examination items in the patients of Example 2 were measured.
[0216] (test methods)
[0217] Using a large-scale multi-item automatic analyzer (Automatic Analizer 7600-020s manufactured by Hitachi), the following inspection items before and after administration were measured. From the values (mean values) before and after administration shown below, it is clear that in the administration method of the medicament of the present invention, after the administration of edaravone, there is no abnormal increase in the clinical examination value, and there is no abnormality in safety. question.
[0218] Check item
Before administration
after administration
GOT(IU / I)
21.3
19.1
GPT(IU / I)
22.2
19.6
γ-GTP (IU / I)
31.6
25.3
BUN (mg / dl)
14.1
14.3
Creatinine (mg / dl)
0.6
0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com