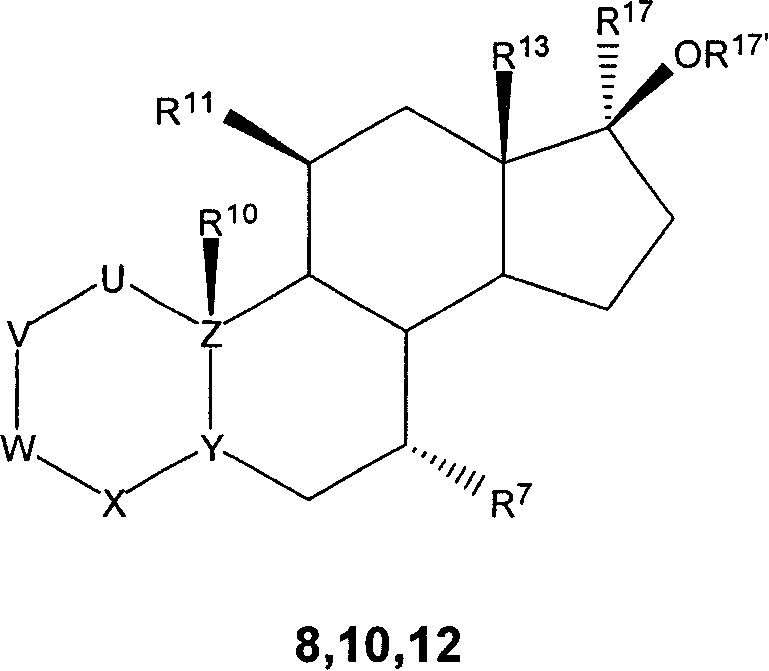
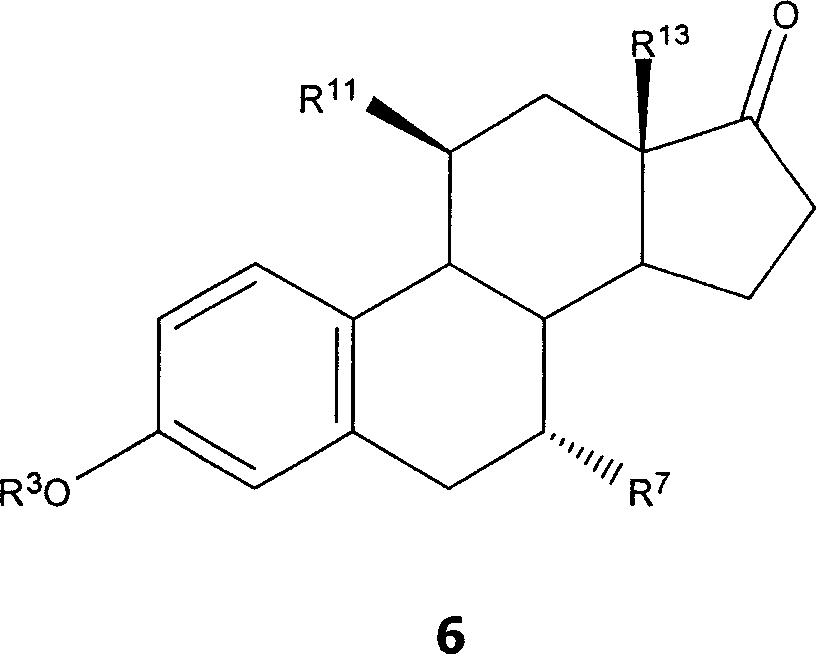
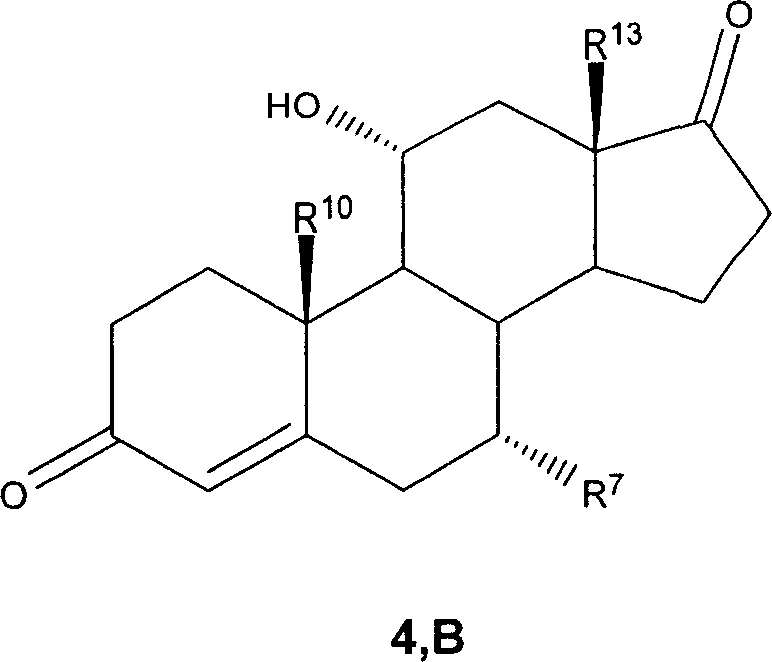
7 alpha-substituted 11 alpha-hydroxysteroids and its microbiological production method
A technique of steroids and pharmacology, applied in the field of 7α-substituted 11α-hydroxy steroids and their microbiological preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124]
[0125] The inclined bacillus culture of Gnomonia cingulata bacterial strain (CBS 15226) was inoculated in a container containing 3% by weight of glucose, 1% by weight of corn steep liquor, 0.2% by weight of NaNO 3 , 0.1 wt% KH 2 PO 4 , 0.2 wt% K 2 HPO 4 , 0.05% by weight KCl, 0.05% by weight MgSO 4 ·7H 2 O and 0.002 wt% FeSO 4 ·7H 2 O (pH 6.0) in 1000 ml of nutrient solution autoclaved at 121° C. for 30 minutes in a 2-liter Erlenmeyer flask at 28° C. on a rotary shaker at 165 rpm for 72 hours. After such preculture, it was inoculated in a 20 liter fermenter covered with 19 liters of sterile medium having the same final composition as the preculture described above. In addition, another 1.0 ml of silicone oil and 1.0 ml of synperonic (oxoalcoholethoxylate) were added to reduce foaming. After a 12-hour growth period at an overpressure of 0.7 bar at a temperature of 28°C, aeration at 20 l / min, stirring at 250 rpm and addition of 4.0 g of 17β-hydroxy-7α-methyle...
Embodiment 2
[0127]
[0128] The Incline bacillus culture of Glomerella cingulata strain (IFO 6425) was inoculated in a container containing 3% by weight of glucose, 1% by weight of corn steep liquor, 0.2% by weight of NaNO 3 , 0.1 wt% KH 2 PO 4 , 0.2 wt% K 2 HPO 4 , 0.05% by weight KCl, 0.05% by weight MgSO 4 ·7H 2 O and 0.002 wt% FeSO 4 ·7H 2 O (pH 6.0) in 1000 ml of nutrient solution autoclaved at 121° C. for 30 minutes in a 2-liter Erlenmeyer flask at 28° C. on a rotary shaker at 165 rpm for 72 hours. After such preculture, it was inoculated in a 20 liter fermenter covered with 19 liters of sterile medium having the same final composition as the preculture described above. In addition, another 1.0ml of silicone oil and 1.0ml of synperonic were added to reduce foaming. After a 12-hour growth period at an overpressure of 0.7 bar at a temperature of 28°C, aeration at 10 l / min, stirring at 350 rpm and addition of 2.0 g of 17β-hydroxy-7α-methylestr-4-en-3-one 30ml of DMF solutio...
Embodiment 3
[0131]
[0132] The culture of Aspergillus ochraceae (CBS 13252) was inoculated in a culture chamber containing 3% by weight of glucose, 1% by weight of corn steep liquor, 0.2% by weight of NaNO 3 , 0.1 wt% KH 2 PO 4 , 0.2 wt% K 2 HPO 4 , 0.05% by weight KCl, 0.05% by weight MgSO 4 ·7H 2 O and 0.002 wt% FeSO 4 ·7H 2 O (pH 6.0) in 500 ml of nutrient solution autoclaved at 121° C. for 30 minutes in a 2 L Erlenmeyer flask, shaken at 28° C. on a rotary shaker at 165 rpm for 72 hours. After such preculture, it was reinoculated in a 10-liter fermentor covered with 9.5 liters of sterile medium having the same final composition as the preculture described above. In addition, another 0.5ml of silicone oil and 0.5ml of synperonic were added to reduce foaming. After a 6-hour growth period at an overpressure of 0.7 bar and a temperature of 28°C, aeration at 5 liters / min, stirring at 350 rpm and addition of 1.0 g of 7α-methylestr-4-ene-3,17-dione 15ml DMF solution. Continue to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com