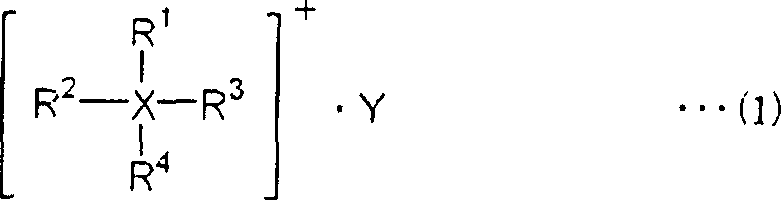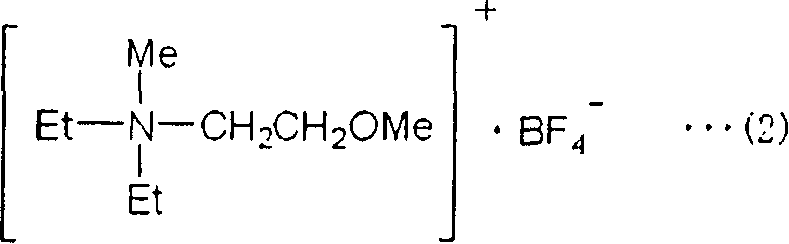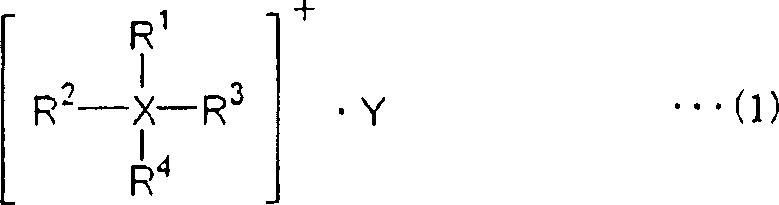Electric double layer capacitor
An electric double layer capacitor and electrolyte technology, applied in electrolytic capacitors, capacitors, hybrid capacitors, etc., can solve the problems of reduced electrical conductivity, narrow potential window, difficult operation, etc., and achieve the effect of excellent charging and discharging performance and reducing internal impedance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0085] Hereinafter, the present invention will be described more specifically by giving synthesis examples, examples, and comparative examples, but the present invention is not limited to the following examples.
Synthetic example 1
[0086] [Synthesis Example 1] Synthesis of Compound (2)
[0087]
[0088] Mix 100 ml of diethylamine (manufactured by Kanto Chemical Co., Ltd.) and 85 ml of 2-methoxyethyl chloride (manufactured by Kanto Chemical Co., Ltd.), put the resulting mixed solution in an autoclave, and react at 100°C 24 hours. At this time, the internal pressure is 0.127MPa (1.3kgf / cm 2 ). After 24 hours, 200 ml of an aqueous solution in which 56 g of potassium hydroxide (manufactured by Katayama Chemical Industry Co., Ltd.) was dissolved was added to the mixture of the precipitated crystals and the reaction solution, and the organic layer separated into two layers was separated using a separating funnel. Furthermore, 100 ml of dichloromethane (manufactured by Wako Pure Chemical Industries, Ltd.) was added, and extraction operation was performed twice. The separated organic layers were combined, washed with saturated brine, dried by adding potassium carbonate (manufactured by Wako Pure Chemical I...
Synthetic example 2
[0091] [Synthesis Example 2] Synthesis of Compound (11)
[0092]
[0093] 100 ml of 2.0 M dimethylamine-THF solution (manufactured by Aldrich) and 9.1 ml of 2-methoxyethyl chloride (manufactured by Kanto Chemical Co., Ltd.) were mixed and reacted in an autoclave at 100° C. for 12 hours. At this time, the internal pressure is 0.36MPa (3.7kgf / cm 2 ). After 12 hours, crystals formed in the reaction solution were removed by filtration, and the filtrate was distilled to remove most of THF to obtain a transparent liquid of a dimethyl-2-methoxyethyl mixture.
[0094] To this liquid was added 8.0 ml of methyl iodide (manufactured by Wako Pure Chemical Industries, Ltd.) under ice-cooling, and the ice bath was removed, followed by stirring overnight. The obtained reactant was distilled under reduced pressure to obtain 3.04 g of oily 2-methoxyethylethyldimethylammonium iodide.
[0095] Then, weigh 2.28g of silver tetrafluoroborate, add 30ml of mixed solvent of chloroform: acetonitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com