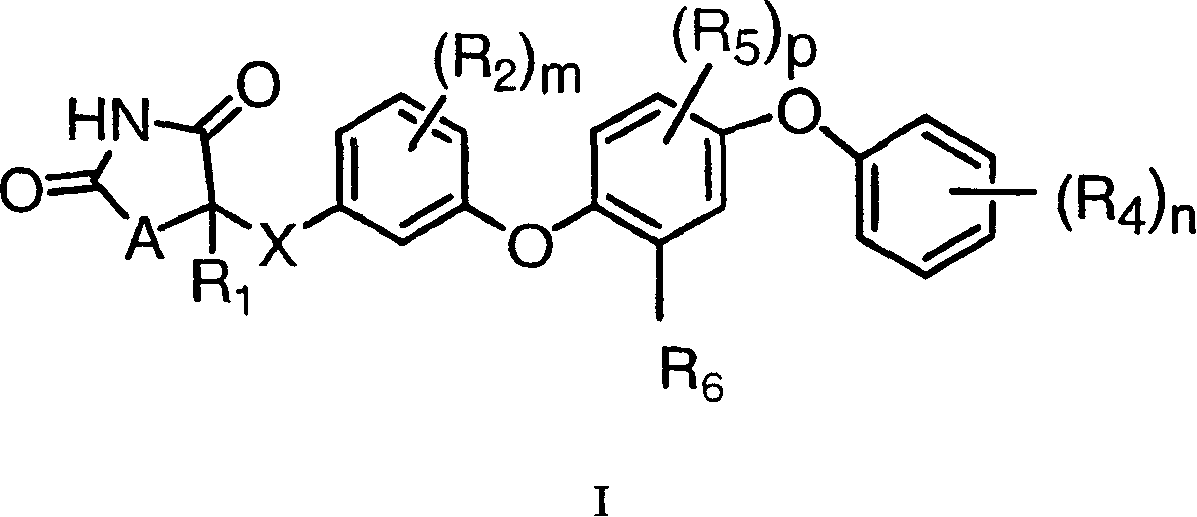
Antidiabetic oxazolidinediones and thiazolidinediones
A technology of alkyl and compound, which is applied in the field of anti-diabetic oxazolidinedione and thiazolidinedione, which can solve the problems of limited curative effect, adverse effect on blood lipid profile, and inability to significantly improve lipid metabolism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 20
[0153] For binding to PPARδ, an aliquot of the receptor was treated with 0.1% nonfat dry milk and 2.5 nM [ 3 h 2 ] L-783483 (17Ci / mmole) in TEGM (10mM Tris, pH7.2, 1mM EDTA, 10% glycerol, 7μL / 100mL β-mercaptoethanol, 10mM sodium molybdate, 1mM dithiothreitol, 5μg / mL pH Peptidase, 2 μg / mL leupeptin, 2 μg / mL benzamidine, and 0.5 mM PMSF), and according to Berger et al. in Novel peroxisome proliferator-activated receptor (PPARγ) and PPARδligands product distinct biological effects.J.Biol.Chem (1999), 274:6718-6725 to increase or decrease the test compound. L-783483 is Example 20 in WO97 / 28137: 3-Chloro-4-(3-(7-propyl-3-trifluoromethyl-6-benzene-[4,5]-isoxazolyloxy base) propylthio) phenylacetic acid. Assay samples were incubated at 4°C for approximately 16 hr in a final volume of 150 μL. Unbound ligand was removed by incubating 100 μL of dextran / gelatin-coated activated charcoal for about 10 min under ice-cold conditions. After centrifugation at 3000 rpm for 10 min at 4°C, 5...
Embodiment 1
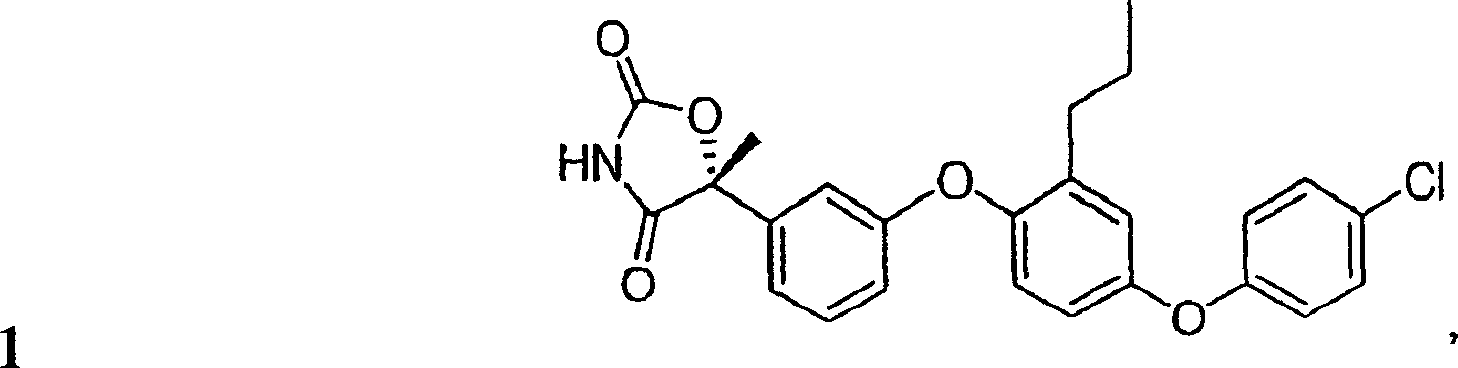
[0253] (5R)-5-{3-[4-(4-Chlorophenoxy)-2-propylphenoxy]phenyl}-5-methyl-1,3-oxazolidine-2,4 - dione
[0254]
[0255] Step 1. Preparation of methyl (2R)-2-{3-[4-(4-chlorophenoxy)-2-propylphenoxy]phenyl}-2-hydroxypropionate
[0256] For intermediate 1 (2.6g, 10mmol), intermediate 9 (3.9g, 15mmol), palladium acetate (90mg, 0.04mmol), bis(tert-butyl)(2-diphenyl)phosphine (179mg, 0,06mmol ) and potassium phosphate (4.2 g, 20 mmol) in toluene (30 mL) were degassed and 2 heated at 100°C for 16 hours. The reaction mixture was diluted with ether (50 mL) and filtered through a short pad of silica gel to afford the crude title product which was used directly in the next step.
[0257] Step 2. Preparation of (2R)-2-{3-[4-(4-chlorophenoxy)-2-propylphenoxy]phenyl}-2-hydroxypropionamide
[0258] A solution of the crude product from step 1 in methanol (35 mL) was cooled to 0°C and saturated with ammonia gas. The solution was concentrated after storage at 25°C for 2 days. The residue ...
Embodiment 2
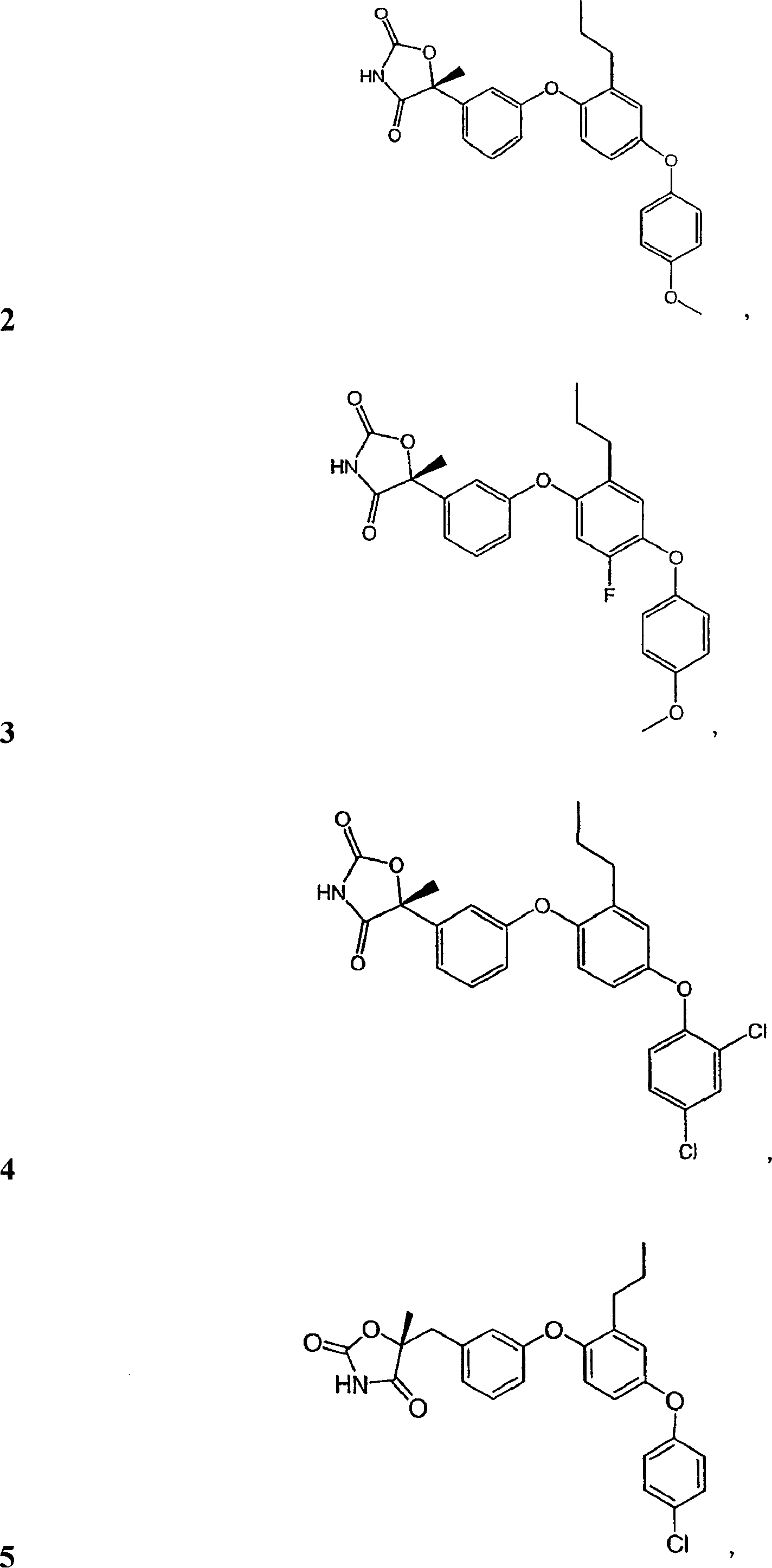
[0264] (5R)-5-{3-[4-(4-Methoxyphenoxy)-2-propylphenoxy]phenyl}-5-methyl-1,3-oxazolidine-2, 4-diketone
[0265]
[0266] The preparation method of the title compound is the same as that of step 1-3 of Example 1, except that intermediate 9 is replaced by intermediate 10 in the first step.
[0267] 1 HNMR (600MHz, CD 3 OD) δ7.26(t, J=7.8Hz, 1H), 7.19(d, J=7.8Hz, 1H), 7.07(t, J=1.8Hz, 1H), 6.94(dd, J=8.4Hz, 2.4 Hz, 1H), 6.93(d, J=12.0Hz, 2H), 6.91(dd, J=7.2Hz, 2.4Hz, 1H), 6.83(d, J=3.6Hz, 1H), 6.82(d, J= 1.8Hz, 1H), 6.74(dd, J=8.4Hz, 2.4Hz, 1H), 6.72(dd, J=8.0Hz, 3.0Hz, 1H), 3.77(s, 3H), 2.47(t, J=7.8 Hz, 2H), 1.69 (s, 3H), 1.54 (m, 2H), 0.87 (t, J = 7.8 Hz, 3H).
[0268] MS (ESI, m / z): 447.9 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com