Cytotoxicity mediation of cells evidencing surface expression of cd44
A cytotoxic, mediated technology, applied in the field of diagnosis and treatment of cancer diseases, can solve the problems affecting the growth and metastasis of tumors in rats
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
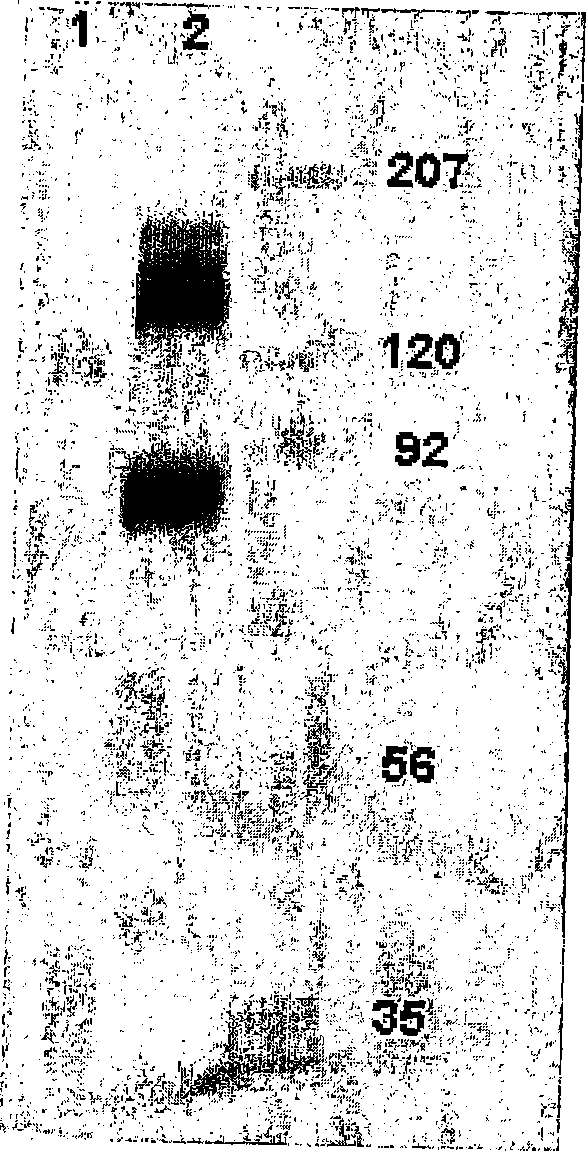
[0082] Identification of binding proteins by Western blotting
[0083] In order to recognize the antigen recognized by the H460-16-2 antibody, the cell membrane expressing the antigen was subjected to gel electrophoresis and transferred to the membrane. Use Western blotting to determine the protein detected by the antibody.
[0084] 1. Cell membrane preparation
[0085] Previous work confirmed the binding of H460-16-2 to breast cancer cell lines MDA-MB-231 (MB-231) and MDA-MB-468 (MB-468) through FACS. Therefore, membrane preparation from these two cell lines was used for antigen recognition. The total cell membrane was prepared from the confluent culture of MB-231 or MB-468 breast cancer cells. Remove the medium from the flask and wash the cells 3 times with PBS. After the last wash, the cells were digested with digestion buffer (Gibco-BRL; Grand Island, NY) at 37°C for 5 minutes. The cells were collected and centrifuged at 1200 rpm for 10 minutes at 4°C. After centrifugation, th...
Embodiment 2
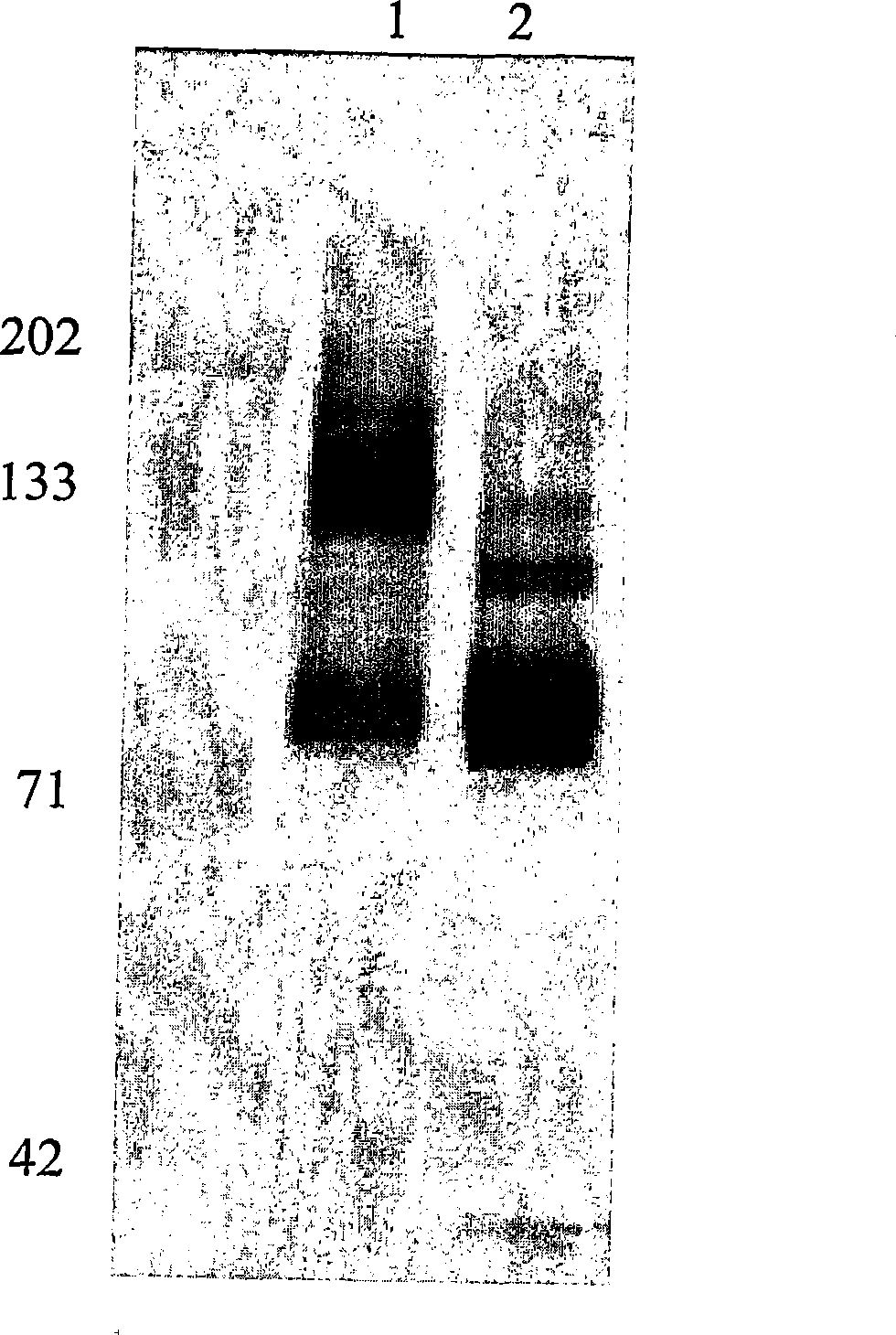
[0095] Determine the glycosylation of the antigen bound to H460-16-2
[0096] In order to determine whether the antigen recognized by antibody H460-16-2 is a glycoprotein, according to the manufacturer's manual (DeglycoPro deglycosylation kit; Prozyme, San Leandro, CA), MB-231 membrane and PNGase F, endo-o -Glucosidase and sialidase A are placed together at room temperature or 37°C for 24 hours. The membrane was separated by 1D polyacrylamide gel electrophoresis as described above and then Western blotting with H460-16-2 as described above.
[0097] Figure 5 The results of Western blotting are shown in. In the MB-468 membrane without glycosidase treatment, H460-16-2 recognized the expected 85-95kD band ( Figure 5 , Panel A, Lane 2). In the membrane treated with glycosidase at 25°C, the band has a clear shift to low molecular weight (lane 4). In membranes treated with glycosidase at 37°C, the binding of H460-16-2 was eliminated (lane 3). In order to determine the completeness of...
Embodiment 3
[0100] Recognition of H460-16-2 bound antigen
[0101] 1. Immunoprecipitation
[0102] Wash 1 mL of Protein GDynabeads (DYNAL) 3 times with 0.1M sodium phosphate buffer of pH 6.0. 2500μg of H460-16-2 was added to the washed beads with a total volume of 500μL. The mixture was left and mixed gently for 1 hour. To remove unbound antibodies, the H460-16-2 coated beads were washed 3 times with 2.5 mL of 0.1 M sodium phosphate buffer containing 0.1% Tween-20, pH 7.4. The H460-16-2 coated beads were washed twice with 5 mL of 0.2 M triethanolamine pH 8.2, and then 5 mL was added. In the presence of 5 mL of triethanolamine containing 20 mM dimethyl pimelimidate at pH 8.2, H460-16-2 was chemically cross-linked with the beads by gentle mixing for 30 minutes. The reaction was stopped by adding 5 mL of 50 mM Tris, pH 7.5. After standing for 15 minutes, the H460-16-2 cross-linked beads were washed 3 times with PBS containing 0.1% Tween-20. The H460-16-2 cross-linked beads were pre-eluted by pla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com