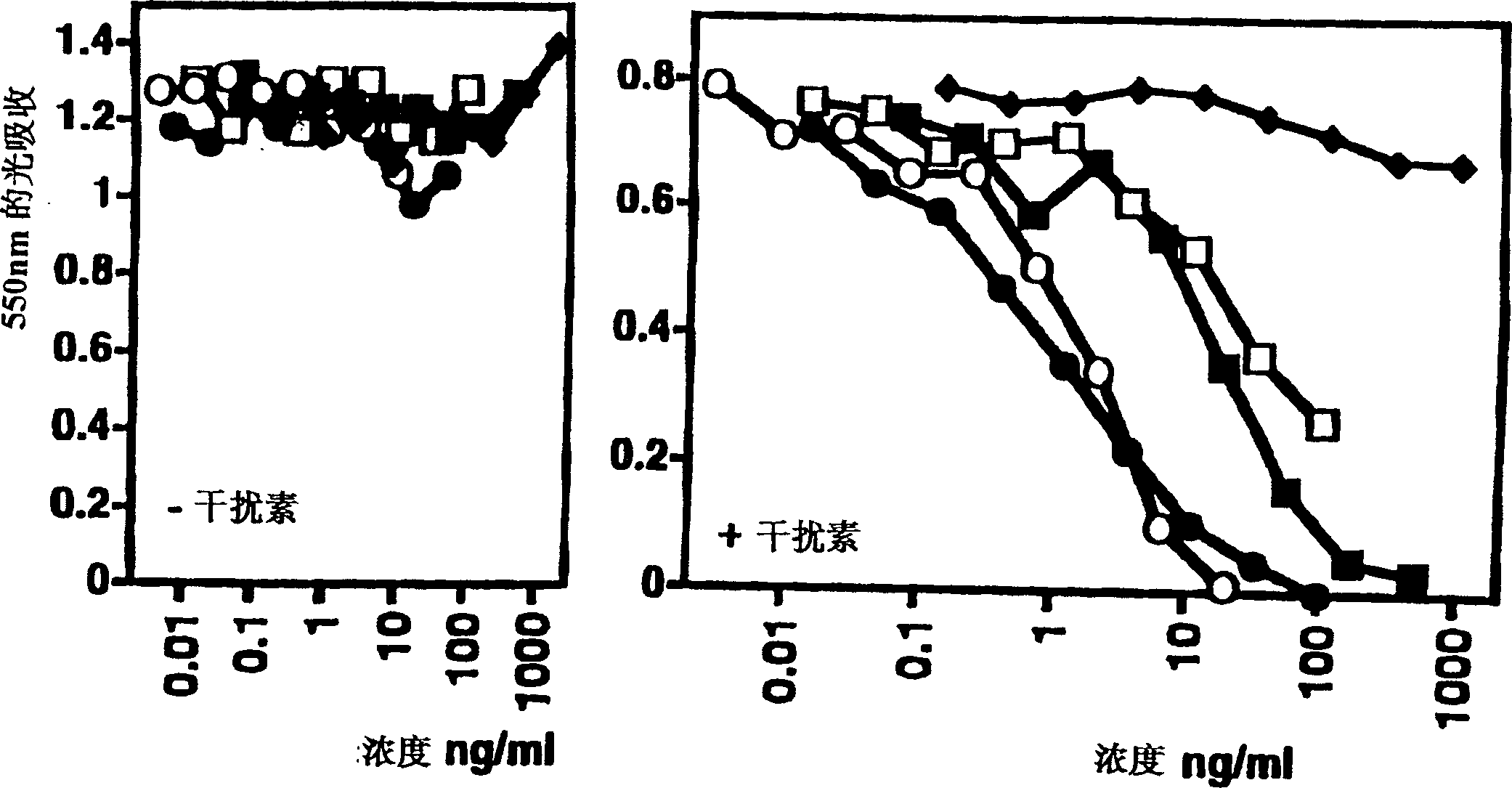
Anti-lymphotoxin-beta receptor antibodies, pharmaceutical composition containing the same and pharmaceutical application thereof
A composition and antibody technology, applied in the field of lymphotoxin heteropolymer complexes, anti-lymphotoxin-β receptor antibodies to enhance the cytotoxicity of tumor cells, and anti-lymphotoxin-β receptor antibodies, which can solve ligands problems such as inability to combine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0159] Preparation of supernatants of baculovirus-infected insect cells containing LT-α / β forms
[0160] Recombinant baculoviruses encoding either full-length LT-[alpha] or a secreted form of LT-[beta] were prepared as described (Crowe et al., Science, 264, PP707-710 (1994)). Take 2×10 5 Density of cells / ml High five insect cells (Invitrogen, San Diego, CA) were inoculated into 7.2 liters of serum-free SF900-II (Gibco) medium. After 48 hours the culture reached 1.8 × 10 6 Cells / ml, use 150ml (3×10 8 PFU / ml) LT-β and 300ml LT-α baculovirus stock solution infection. Cultures were harvested after 2 days and cellular debris removed by centrifugation. EDTA and PMSF (final concentrations of 1 mM EDTA and 150 [mu]M PMSF) were added and the clarified supernatant was concentrated 10-fold by ultrafiltration using a SIYM10 (Amicon) spiral column. The concentrate was divided into 6 aliquots of 120 ml each and aliquots were stored at -70°C until further purification.
example 2
[0162] Preparation of soluble LT-β receptors as immunoglobulin Fc chimeras
[0163] The extracellular domain of LT-β-R up to the transmembrane region was amplified from cDNA clones by PCR using primers that incorporated NotI and SalI restriction enzyme sites at their 5' and 3' ends, respectively (Browning et al., J. . Immunol., 154, PP. 33-46 (1995)). The amplified product was cleaved with NotI and SalI, purified, and ligated together with the SalI-NotI fragment encoding the Fc fragment of human IgG1 into the vector pMD901 linearized by NotI. The resulting vectors contained the dihydrofolate reductase gene and the LT-β-R-Fc chimera driven by different promoters. The vector was introduced into CHO dhfr- cells by electroporation, and methotrexate-resistant clones were isolated by standard methods. LT-β-R-Fc was secreted into the culture medium, and cell lines producing large amounts of the chimeric protein were selected by ELISA assay. Grow high-producing cell lines to larger...
example 3
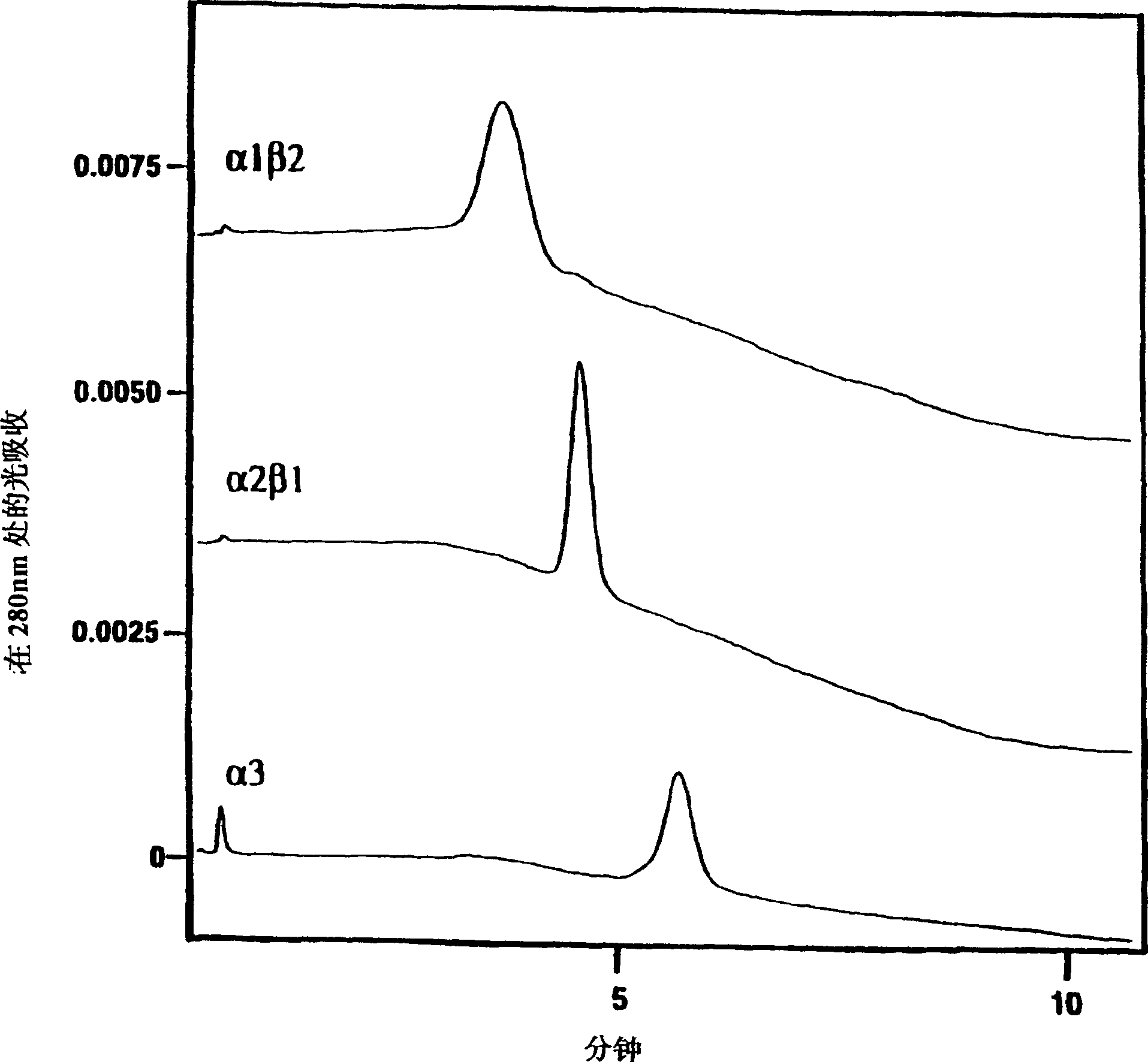
[0165] Affinity chromatography of LT-α1 / β2 with TNF-R and LT-β-R
[0166] To prepare receptor for affinity purification of the LT form of receptor, LT-β-R-Fc (as described in Example 2 herein) and TNF-R A purified preparation of p60-Fc (Crowe et al., Science, 264, pp. 707-10 (1994)) was immobilized on CNBr-Sepharose (Pharmacia). The resin was subjected to one round of elution prior to use. A portion (120 ml) of the SIY10 concentrate was passed through two consecutive LT-α and LT-α2 / β1 bound p60TNF-R-Fc columns. The flow-through material containing LT-α1 / β2 and LT-β was passed through a LT-β-R-Fc column. Wash the column with 5 column volumes of PBS, PBS containing 0.5M NaCl, and PBS, respectively, and then use 25 mM sodium phosphate, 100 mM NaCl, pH 3.5 to elute LT-α and LT-α2 / β1 complexes. The eluted fractions were immediately eluted with 1 / 20 volume of 0.5M sodium phosphate, pH 8.6, and kept on ice. Protein-containing fractions were identified by absorbance at 280 nm, pea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com