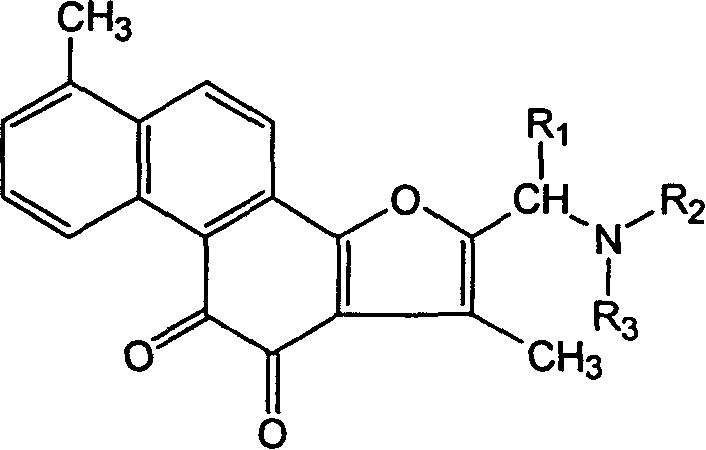
Tanshinone I derivatives and pharmaceutical application thereof
A derivative, the technology of tanshinone, applied in the field of medicine, can solve the problems that the molecular structure is difficult to modify, and the medicinal value of tanshinone I has not been fully developed, so as to improve bioavailability, bioavailability and curative effect, and biological Effect of Utilization and Enhancement of Efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1. 2-(cyclohexylamine) methyl-tanshinone I synthesis steps and structural confirmation
[0053] Mix 1.46g of tanshinone I, 0.5mL of 37% formaldehyde solution, 2.5g of cyclohexylamine, and 50mL of acetic acid, heat and reflux in an oil bath for 10 hours, remove the solvent from the mixture under reduced pressure to obtain a red solid, dissolve it in water, filter, and add alkali to the filtrate to adjust the pH to 9, filtered, the filter cake was dried, and separated by silica gel column chromatography (eluent: chloroform / methanol=15 / 1) to obtain 1.14 g (yield 55%) of the expected compound.
[0054] 1 H-NMR (CDCl 3 )δ9.13(d, 1H), 8.20(d, 1H), 7.85(d, 1H), 7.57(q, 1H), 7.24(d, 1H), 3.83(s, 2H), 3.21~3.38(m , 4H), 2.75(s, 3H), 2.31(s, 3H), 1.75~1.81(m, 4H), 1.52~1.69(m, 4H).
Embodiment 2
[0055] Example 2. 2-(2', 5'-dimethylpiperazine) methyl-tanshinone I synthesis steps and structural confirmation
[0056]Mix 1.46g of tanshinone I, 0.5mL of 37% formaldehyde solution, 2.8g of 2'5-dimethylpiperazine, and 50mL of acetic acid, and heat to reflux in an oil bath for 10 hours. Dissolve, filter, add alkali to the filtrate to adjust the pH to 9, filter, dry the filter cake, and separate by silica gel column chromatography (eluent: chloroform / methanol=15 / 1) to obtain 1.08 g (yield 51%) of the expected compound.
[0057] 1 H-NMR (CDCl3) δ9.28(d, 1H), 8.28(d, 1H), 7.85(d, 1H), 7.58(q, 1H), 7.25(d, 1H), 3.38~3.51(m, 2H ), 2.92~3.14(m, 4H), 3.73(s, 2H), 2.67(s, 3H), 2.28(s, 3H), 1.82(s, 1H), 1.52(s, 3H), 1.31(s, 3H).
Embodiment 3
[0058] Example 3. 2-(diallylamino)methyl-tanshinone I synthesis steps and structural confirmation
[0059] Mix 1.46g of tanshinone I, 0.5mL of 37% formaldehyde solution, 2.4g of diallylamine, and 50mL of acetic acid, heat and reflux in an oil bath for 10 hours, remove the solvent from the mixture under reduced pressure to obtain a red solid, add water to dissolve, filter, and add alkali to the filtrate Adjust the pH to 9, filter, dry the filter cake, and separate by silica gel column chromatography (eluent: chloroform / methanol=15 / 1) to obtain 1.03 g (yield 50%) of the expected compound.
[0060] 1 H-NMR (CDCl3) δ9.26(d, 1H), 8.27(d, 1H), 7.91(d, 1H), 7.48(q, 1H), 7.22(d, 1H), 5.42~5.58(m, 2H ), 4.75~4.91(m, 4H), 3.85(s, 2H), 2.81~2.98(m, 4H), 2.61(s, 3H), 2.12(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com