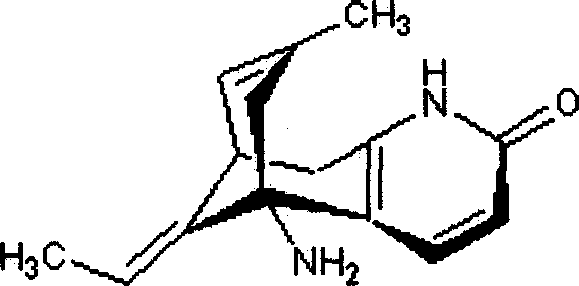
Huperzine-A oral cavity disintegration tablet and its preparation method
The technology of oral disintegrating tablet and huperzine A is applied in the field of oral disintegrating tablet of huperzine A and its preparation, which can solve the problems such as being unfavorable for the elderly to take, unfavorable for senile dementia patients and the like, and achieves good results, The preparation process is simple and the effect of good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: preparation embodiment
[0027] Huperzine A 0.05 g, Microcrystalline Cellulose Blank Ball Core 40.0 g, Eudradit E-100 1.0g, mannitol 60g, low-substituted hydroxypropyl cellulose 3.5g, cross-linked polyvinylpyrrolidone 4.7g, microcrystalline cellulose 5.9g, aspartame 0.5g, orange essence 0.5g, stearic acid Magnesium 1.0 g, appropriate amount of PVP-K30 and ethanol solution.
[0028] Preparation of huperzine A drug-containing microspheres:
[0029] Will Eudradit Dissolve E-100 in 30ml of ethanol solution and fully stir to dissolve it, and use it as a coating solution for later use;
[0030] Dissolving huperzine A in the above-mentioned coating solution to make a drug-containing coating solution;
[0031] Place the microcrystalline cellulose blank core in a coating pan, and coat the drug-containing coating solution evenly on the blank core to prepare drug-containing microspheres.
[0032] To prepare mannitol granules:
[0033] Take mannitol, use 2% ...
Embodiment 2
[0035] Embodiment 2: stability test
[0036] Take the orally disintegrating tablet prepared in Example 1, spread the tablet on a plate, and carry out a strong light irradiation test (illumination: 4500lx±500lx), and continuously irradiate for 10 days;
[0037] Take the orally disintegrating tablet prepared in Example 1, put it in a watch glass, place it in a thermostat at 60°C±2°C for 10 days, and conduct a high temperature stability test;
[0038] Take the orally disintegrating tablet prepared in Example 1, place it in an environment with a relative humidity of 75%±5% for 10 days, and carry out a high humidity stability test;
[0039] Samples were taken on 10 days to check their properties, disintegration time, dissolution rate, related substances and content. The results are shown in Table 1. The results show that all the indicators of the product stability test are in compliance with the regulations.
[0040] category
Embodiment 3
[0041] Embodiment 3: comparative test
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com