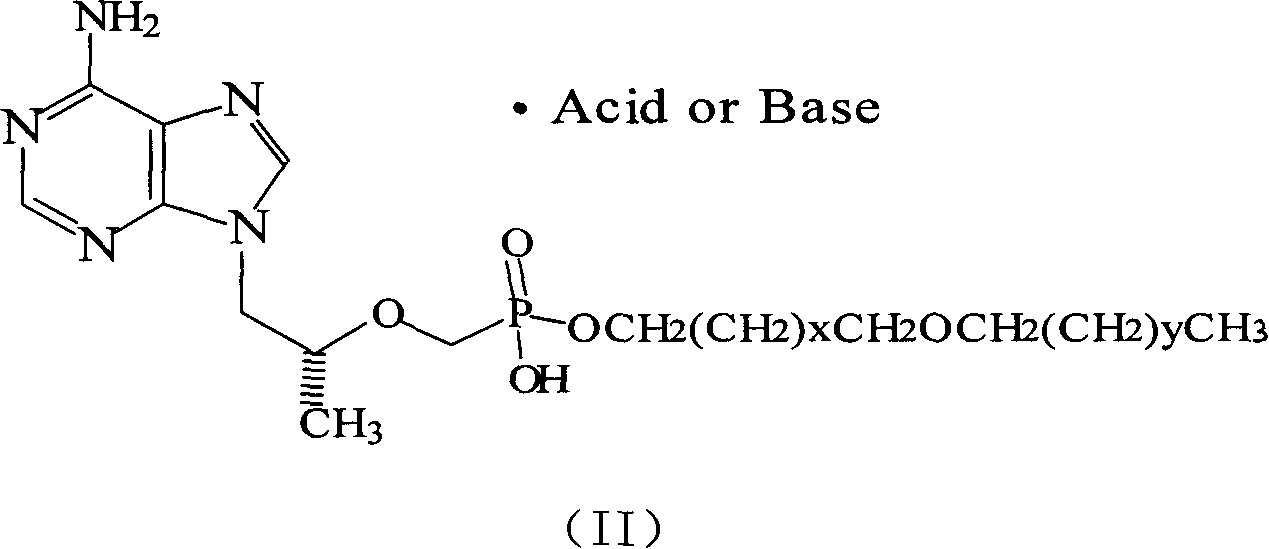
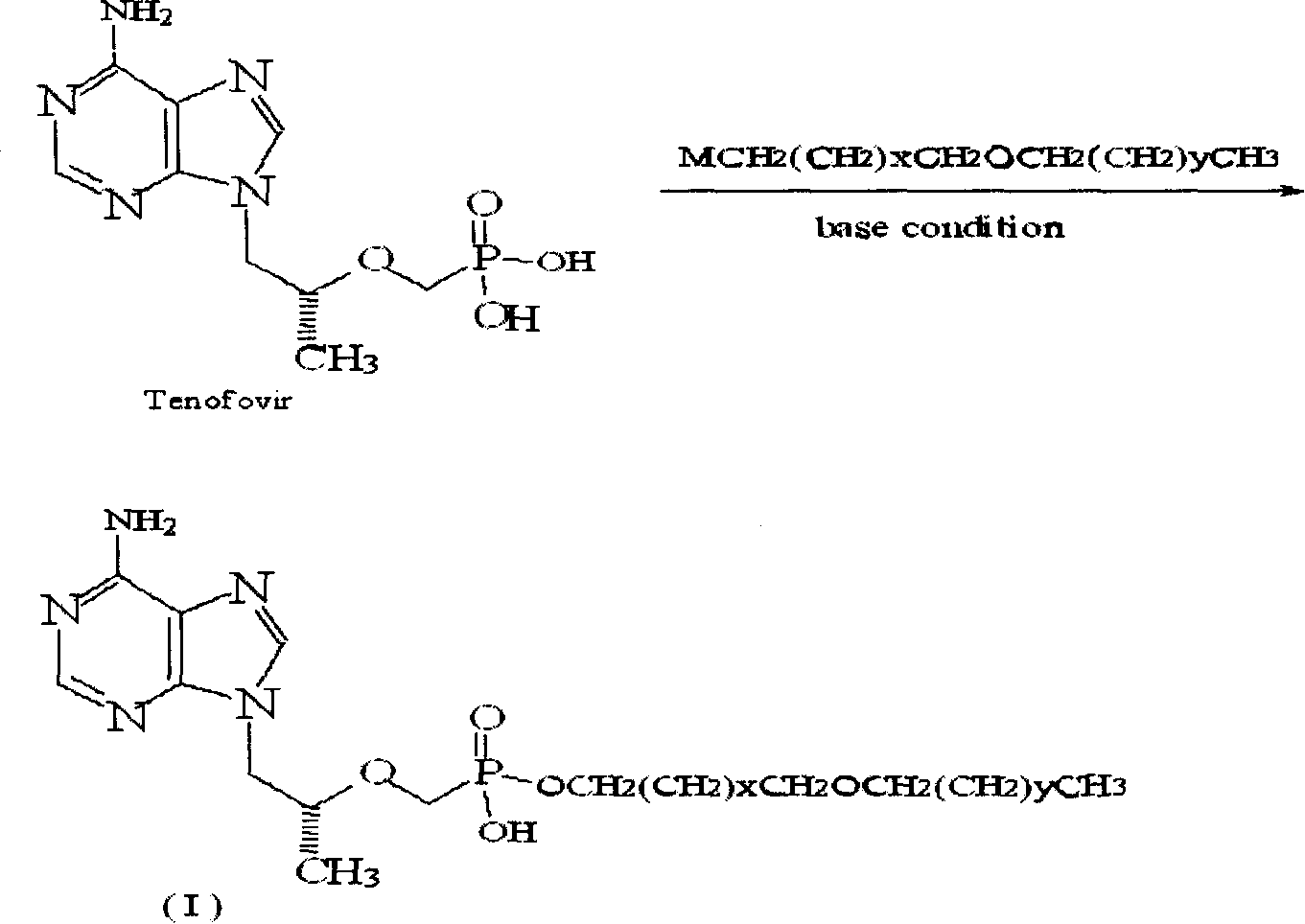
Tenoforv monoester compounds with HIV-1/HBV virus copying inhibiting activity
A tenofovir and virus replication technology, applied in the field of tenofovir monoester compound, can solve problems affecting development and poor bioavailability of tenofovir
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of (R)-9-[2-[(hexadecyloxypropyl)phosphomethoxy]propyl]adenine (5)
[0028] (R)-9-[2-(phosphomethoxy)propyl]adenine 1.44g (PMPA 5.0mmol), DMF20ml, 1-bromo-3-hexadecyloxypropane 1.82g (5.0mol), triethyl Mix 0.61g (6.0mmol) of amine, heat to 80°C and stir for 6h. After spin-drying, a yellow oily substance is obtained. Add 100ml of a mixed solvent of dichloromethane and methanol at a ratio of 1:1 to fully dissolve and filter. The filtrate is spin-dried, and the residue After separation by silica gel column chromatography, 2.04 (71.5%) was obtained as a pale yellow solid. 1 HNMR (DMSO) δ, (ppm): 0.838 (3H, t, CH 3 ), 0.919-0.933 (3H, d, CH 3 ), 1.134-1.225 (26H, m, 13×CH 2 ), 1.414-1.475 (2H, m, CH 2 ), 1.606-1.638 (2H, m, CH 2 ), 3.128-3.416 (6H, m, 3×OCH 2 ), 3.642 (2H, s, OCH 2 P), 3.837-3.850 (1H, m, CH), 4.084-4.269 (2H, m, NCH 2 ), 7.109 (2H, s, NH 2 ), 8.098, 8.345 (2H, s, respectively H on the purine ring).
Embodiment 2
[0029] Embodiment 2: Preparation of (R)-9-[2-[(hexadecyloxypropyl) phosphate methoxy] propyl] adenine fumarate (6)
[0030] Dissolve an equal amount of (R)-9-[2-[(hexadecyloxypropyl)phosphomethoxy]propyl]adenine and fumaric acid in hot isopropanol and stir for 0.5h, then cool and analyze at room temperature. crystal, and the precipitated solid was filtered off and washed with ether to obtain a white solid. 1 HNMR (DMSO) δ, (ppm): 0.833 (3H, t, CH 3 ), 0.910-0.926 (3H, d, CH 3 ), 1.136-1.219 (26H, m, 13×CH 2 ), 1.428-1.476 (2H, m, CH 2 ), 1.601-1.630 (2H, m, CH 2 ), 3.127-3.419 (6H, m, 3×OCH 2 ), 3.642 (2H, s, OCH 2 P), 3.835-3.853 (1H, m, CH), 4.096-4.267 (2H, m, NCH 2 ), 6.63 (2H, s, H on the double bond of fumaric acid), 6.998 (2H, s, NH 2 ), 8.103, 8.339 (2H, s, respectively H on the purine ring).
Embodiment 3
[0031] Example 3: Preparation of (R)-9-[2-[(hexadecyloxypropyl)phosphorylmethoxy]propyl]adenine sodium salt (7)
[0032] An equal amount of (R)-9-[2-[(hexadecyloxypropyl)phosphomethoxy]propyl]adenine and NaOH were dissolved in water, stirred for 0.5 h, and freeze-dried to obtain a white flocculent solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com