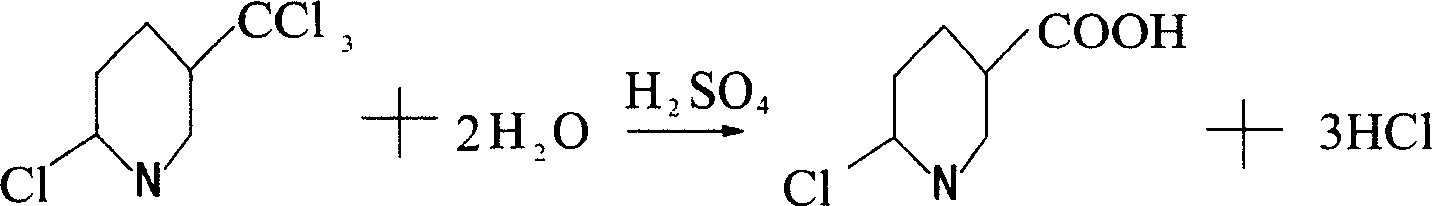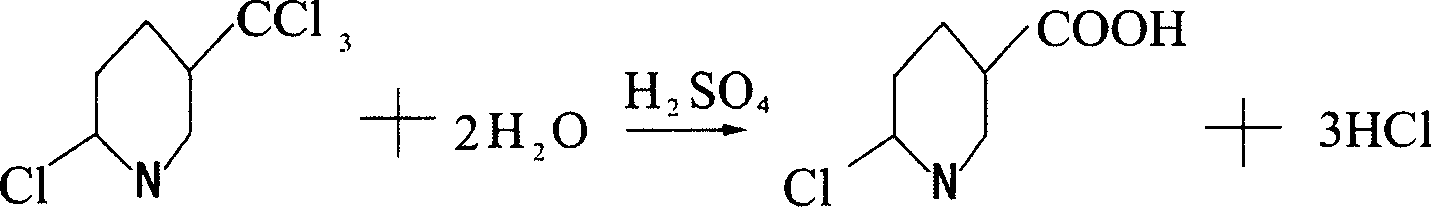Method for preparing 6-chloro-3-pyridinecarboxylic acid
A technology of pyridine carboxylate and its manufacturing method, which is applied in the direction of organic chemistry, etc., can solve the problems of difficulty in improving the purity, difficulty in separation, and high cost of 6-chloro-3-pyridine carboxylate, and achieve the depth of application, high product purity, and low cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Put 600 grams of concentrated sulfuric acid into a 1000ml four-necked flask with a stirrer, thermometer and tail gas outlet pipe, turn on the stirring, and slowly flow 100 grams of 2-chloro-5-trichloromethylpyridine into the flask. Then, the temperature is slowly raised to 120°C. When the temperature reaches 80°C, hydrogen chloride gas begins to be released, and the lye is introduced through the tail gas outlet pipe for absorption. When the temperature reaches 110°C, start to keep the temperature, keep the temperature between 110-120°C for 2 hours to end the reaction, and lower the temperature to 30°C. Then, the reaction solution was slowly added to ice water composed of 1000 grams of ice and 2000 grams of water, and the reaction solution was added while stirring, and a white precipitate was precipitated. After the addition, continue to stir, then filter, and dry to obtain 87.8 g of 6-chloro-3-carboxylic acid pyridine. The purity of the liquid spectrum analysis i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com