Freeze-dried powder-injection of methionine vitamin B1, and its prepn. method
A technology of freeze-dried powder injection and methionine, which is applied in the field of methionine vitamin B1 pharmaceutical preparations, can solve problems such as reduced stability, increased toxic and side effects, and influence on drug efficacy, and achieves good resolubility and reasonable formulation. , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
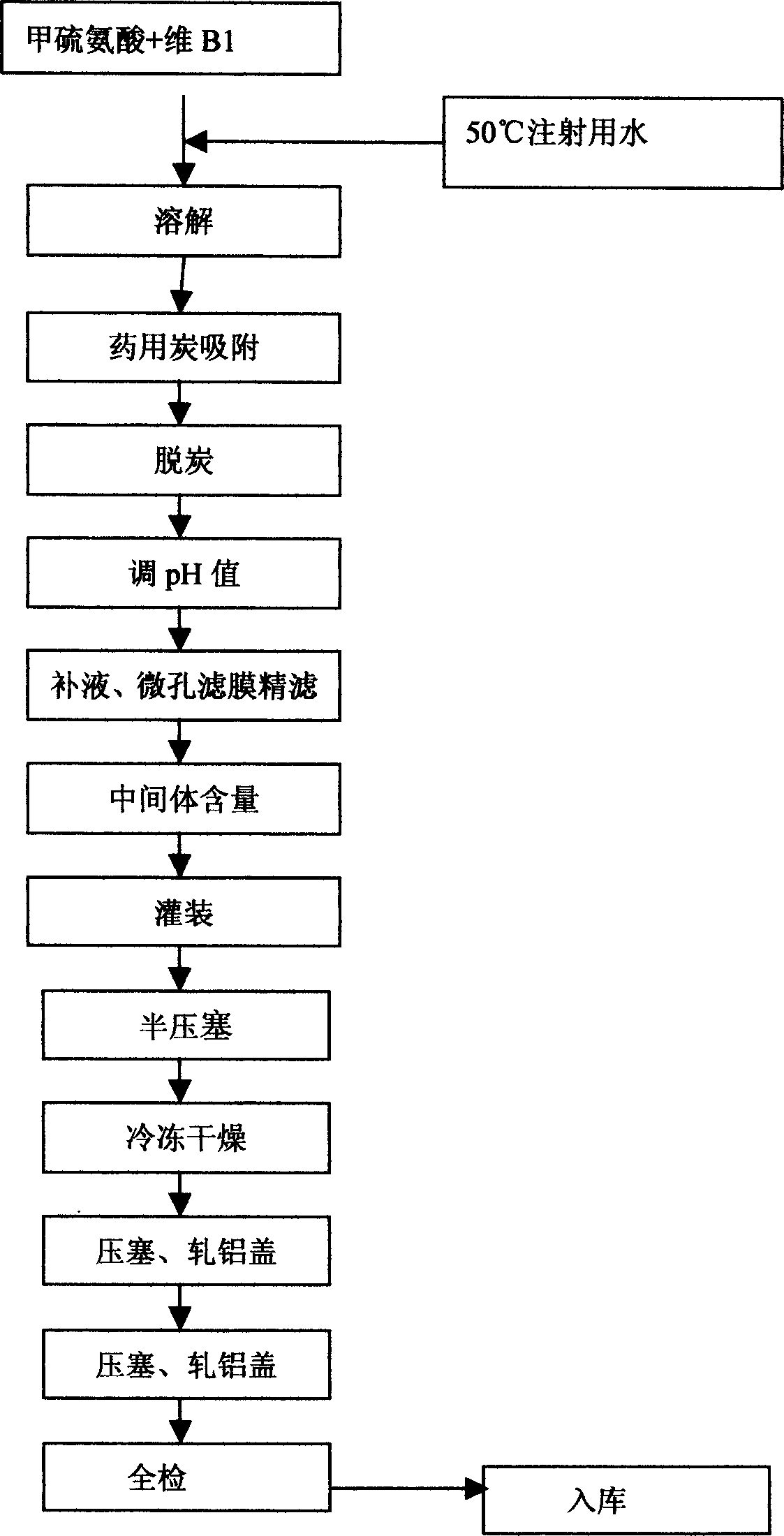
[0038] The preparation process is as follows:
[0039] Take by weighing methionine 40g by embodiment 1 prescription, vitamin B 1 4g, put it in a clean container, add 800ml of water for injection, heat and stir to keep the temperature of the aqueous solution at 45°C, add 0.4g of charcoal for needles, stir for 20 minutes, and decarbonize by coarse filtration. The measured pH of the solution is 4.2, and use 0.1 mol / L sodium hydroxide solution to adjust the pH value to 4.8, add water for injection to the prescription amount, filter with a 0.22 micron microporous membrane, check the intermediate content, then fill the filtrate in a 7ml / support vial, 1ml vial, stoppered, freeze-dried, filled with nitrogen, corked and packaged.
[0040] Methionine
Vitamin B 1
Add water for injection to
100g
10g
2000ml
Co-made
1000 bottles
[0041] The preparation process is as follows:
[0042] Take by weighing methionine 100g by embodime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com