Synthesis method of 2-chloro-3-amido-4picoline from cyanoacetamide and acetone
A technology of picoline method and cyanoacetamide, which is applied in the direction of organic chemistry, can solve the problems of high cost, long process route, and low total yield, and achieve the effect of less reaction steps, good selectivity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
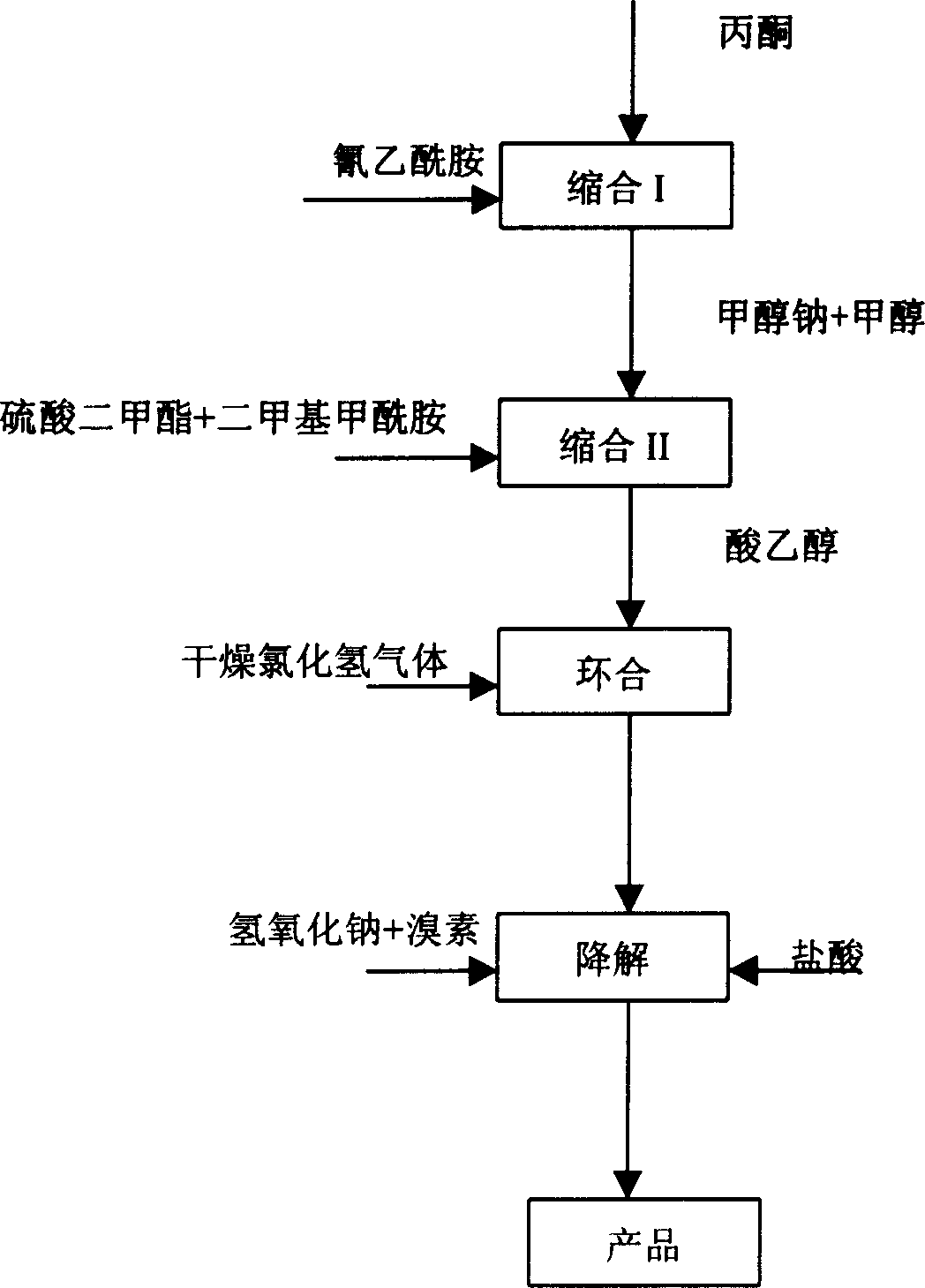
[0032] Step 1: Synthesis of Condensate I
[0033] Add 43.4g (0.5mol) of cyanoacetamide, 80ml of benzene, and 1.3g of piperidine into the reaction kettle, heat up to reflux, dropwise add a mixture of acetone (55g) and glacial acetic acid (9.6g), and reflux to separate water while adding dropwise. After the dropwise addition, continue to reflux and divide the water until all the water evaporates. After the reaction, the temperature was lowered to 25° C., and the layers were washed with 50 ml of water. The organic phase is adjusted to PH value to 8 ~ 9 with saturated sodium carbonate, static layering, the aqueous phase is extracted with 50ml benzene, the combined organic phase is washed once with saturated sodium chloride solution, and concentrated to dryness to obtain 54.5 grams of condensate I (content 91.3%, yield 80.2%). After cooling the condensate I, 20 g of methanol was added as a solvent.
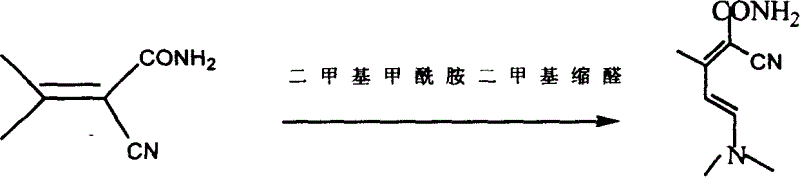
[0034] Step 2: Synthesis of Condensate II
[0035] In a reaction kettle, under...
Embodiment 2
[0043]Change solvent benzene into toluene in the first step of embodiment 1, adjust pH value and replace with saturated sodium bicarbonate solution, other operating conditions (comprising aftertreatment step) are identical with embodiment 1. As a result, 55.5 g of condensate I was obtained (content 92.5%, yield 81.7%). All the other are with embodiment 1.
Embodiment 3
[0045] Change the logical hydrogen chloride gas reaction time into 20 hours in the third step of embodiment 1, change solvent toluene into benzene, and other operating conditions (comprising aftertreatment steps) are identical with embodiment 1. As a result, 44.2 grams of dry cyclized product was obtained (content 90.5%, yield 76.6%). All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com