Phenolphthalein type benzoxazine intermediate and composition and method of making the same
A technology for benzoxazine and intermediates, which is applied in the field of benzoxazine intermediates and compositions and synthesis, can solve the problems of not being able to obtain benzoxazine compounds successfully and have not seen reports, and achieve low price, Effect of increasing crosslink density, improving toughness and impact resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
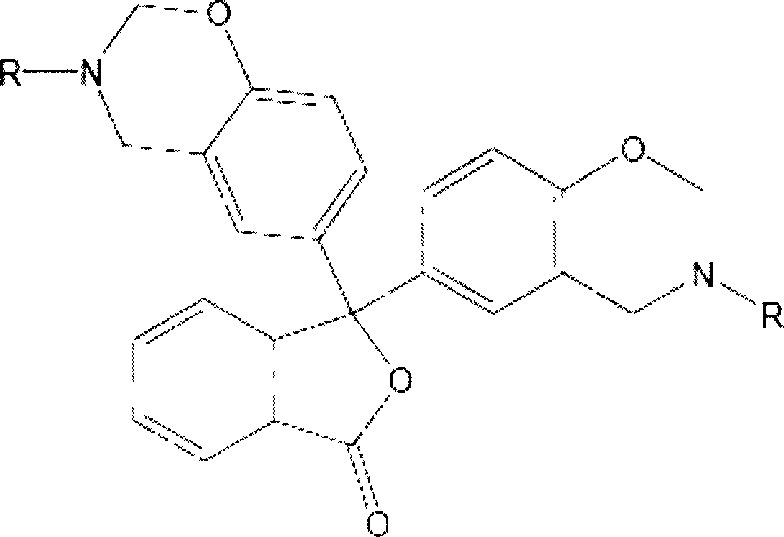
[0038] Preparation of 3,3-bis(3-allyl-3,4-dihydro-2H-1,3-benzoxazine)-1(3H)-isobenzofuranone (referred to as phenolphthalene) by solution synthesis Propylamine benzoxazine), its structural formula is as follows:
[0039]
[0040] Dissolve phenolphthalein in absolute ethanol at room temperature, and add allylamine and paraformaldehyde in sequence at a molar ratio of phenolic hydroxyl, amino, and aldehyde functional groups of 1:1:2. After stirring evenly, the water bath is heated and refluxed. After 2 hours of reaction, the ethanol is distilled off under reduced pressure, cooled and left to stand, and a light yellow translucent viscous body is obtained. After washing, purification, and drying, the phenolphthalein allylamine benzoxazine intermediate is obtained. body with a yield of 79.4%.
[0041] Subsequently, the phenolphthalein allylamine benzoxazine intermediate is cured, and the curing temperature and time are segmented: 1 hour at 120°C, 1 hour at 180°C, 40 minutes at 2...
Embodiment 2
[0043] The phenolphthalein allylamine benzoxazine intermediate is prepared by a solution method, and its operation steps are the same as in Example 1, except that the molar ratio of phenolic hydroxyl, amino, and aldehyde functional groups is added in a molar ratio of 1:1.1:2.2 Phenolphthalein, Allylamine, Formaldehyde solution. Finally, a light yellow transparent viscous body was obtained, namely the phenolphthalein allylamine benzoxazine intermediate, with a yield of 80%.
Embodiment 3
[0045] The solution method is used to prepare phenolphthalein allylamine benzoxazine, and its operation steps are the same as in Example 1, except that the molar ratio of phenolphthalein, olefin and phenolphthalein in the molar ratio of phenolic hydroxyl, amino, and aldehyde functional groups is 1: 1.2: 2.4. Propylamine, formaldehyde solution. Finally, a light yellow transparent viscous body was obtained, namely the phenolphthalein allylamine benzoxazine intermediate, with a yield of 78%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Tg | aaaaa | aaaaa |
| Tg | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com