Stable recombination human interferon alpha 1b water solution
A technology of recombinant human interferon and interferon alpha, applied in the direction of interferon, cytokine/lymphokine/interferon, specific peptide, etc., can solve the problems of long-term storage, instability, etc.
Active Publication Date: 2010-08-18
BEIJING TRI PRIME GENE PHARMA CO LTD
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, when interferon exists in the state of aqueous solution, its main disadvantage is that it is unstable and cannot be stored for a long time
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
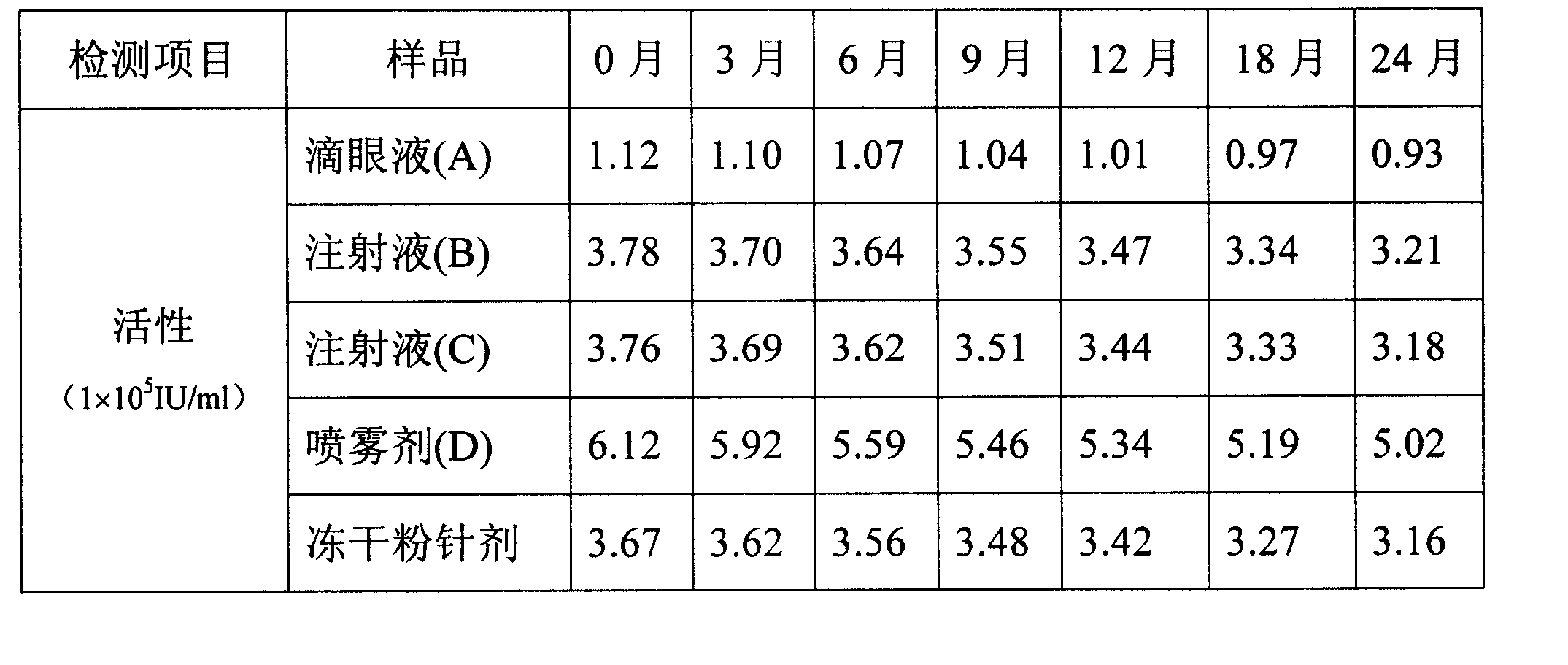
The invention relates to a stabilized recombinant human interferon alpha1b water solution, where the water solution with a total volume of 1000ml contains, therapeutic effective dose of interferon alpha1b, 2.5-27.0g of sodium chloride, 0.15-0.60g of citric acid, 1.5-6.0g of dodecahydro-disodium phosphate, and 5-20g of human blood albumin.
Description
technical field The present invention relates to a stable aqueous solution of interferon. Background technique It has been more than 40 years since interferon was discovered in 1957. So far, great progress has been made in the research on the basic theory, production and preparation technology and clinical application of interferon. Recombinant human interferon α1b is suitable for chronic hepatitis B, hepatitis C, hairy cell leukemia, chronic myelogenous leukemia, condyloma acuminatum, and various infectious diseases and tumors, with a wide range of clinical applications and a large dosage. But this medicine mainly exists in the form of freeze-dried powder injection, needs to add water for injection during use, brings some inconvenience to clinical use, what is more important is that the manufacturing cost of powder injection is higher, and the price is expensive, so many people who need to use interferon It is unaffordable for patients, which severely limits the populari...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07K14/56
Inventor 杨大军刘金毅刘永全孙俭波程永庆
Owner BEIJING TRI PRIME GENE PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com