Novel anthranilamide insecticides
A technology of alkylamino and alkyl, applied in agronomic and non-agronomic fields, can solve the problems of reduced productivity and increased consumer cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0168] The preparation of N-carboxyaminobenzoic anhydrides of formula 6 can be achieved from isatins of formula 9 as shown in scheme 5.
[0169] Route 5
[0170]
[0171] Isatins of formula 9 were obtained from aniline derivatives of formula 8 according to the methods of F.D. Popp, Adv. Heterocycl. Chem. 1975, 18, 1-58 and J.F.M. 12(3), 273-324. Oxidation of isatin 9 with hydrogen peroxide usually gives the corresponding N-carboxyaminobenzoic anhydride 6 in good yields (G. Reissenweber and D. Mangold, Angew. Chem. Int. Ed. Engl. 1980, 19, 222-223 ). N-Carboxyaminobenzoic anhydrides can also be obtained from anthranilic acid 5 by a variety of well known methods involving the reaction of 5 with phosgene or phosgene equivalents.
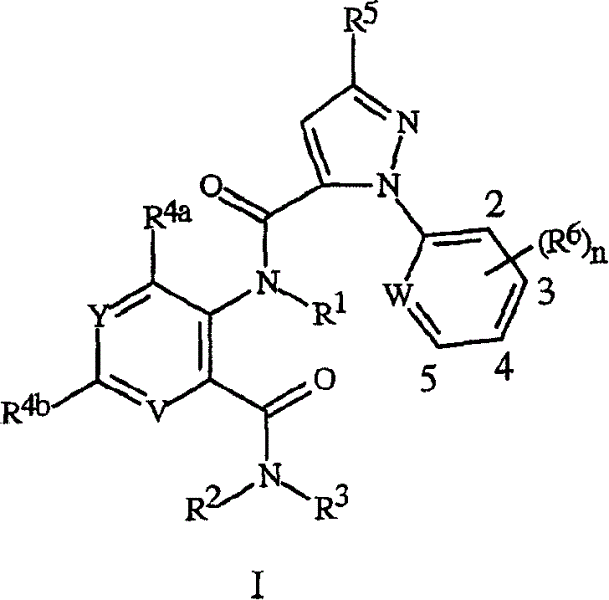
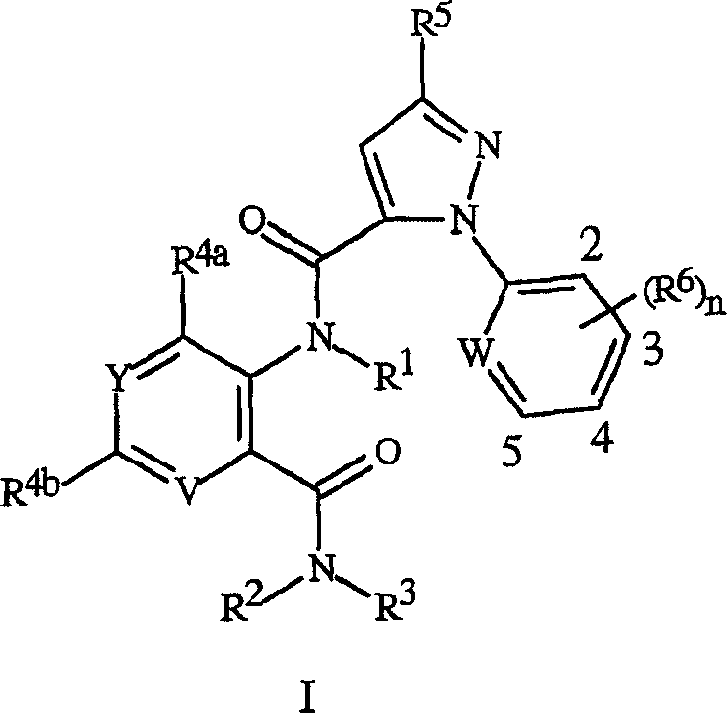
[0172] A variety of R 5 The synthesis of heterocyclic acids of formula 4 for substituents is described in schemes 6-12. Preferred compounds of the invention are derived from pyrazole acids of formula 4 substituted with 3-chloropyridyl. Thus, ...
Embodiment 1
[0201] 1-(3-chloro-2-pyridyl)-N-[2,4-dichloro-6-[(methylamino)carbonyl]phenyl]-3-[(methylsulfonyl) Preparation of O]-1H-pyrazole-5-carboxamide
[0202] Step A: Preparation of ethyl 1-(3-chloro-2-pyridyl)-3-pyrazolidinone-5-carboxylate
[0203] A 2-liter four-neck flask equipped with a mechanical stirrer, thermometer, addition funnel, reflux condenser, and nitrogen inlet was charged with absolute ethanol (250 mL) and sodium ethoxide in ethanol (21%, 190 mL, 0.504 mol). The mixture was heated to reflux at about 83°C. It was then treated with 3-chloro-2-hydrazinopyridine (68.0 g, 0.474 mol). The mixture was reheated to reflux over 5 minutes. The yellow slurry was then treated dropwise with diethyl maleate (88.0 mL, 0.544 mol) over 5 minutes. The reflux rate increased significantly during the addition. At the end of the addition, all starting material had dissolved. The resulting orange solution was kept at reflux for 10 minutes. After cooling to 65°C, the reaction mix...
Embodiment 2
[0218] 1-(3-chloro-2-pyridyl)-N-[2,4-dichloro-6-[[(1-methylethyl)amino]carbonyl]benzene Preparation of base]-3-[(methylsulfonyl)oxy]-1H-pyrazole-5-carboxamide
[0219] To 6,8-dichloro-2-[1-(3-chloro-2-pyridyl)-3-[(methylsulfonyl)oxy]-1H-pyrazol-5-yl]-4H-3,1 - To a solution of benzoxazin-4-one (ie the product of Example 1, Step D) (0.05 g, 0.10 mmol) in acetonitrile (3 mL) was added isopropylamine (0.5 mL, 5.87 mmol) dropwise. The resulting solution was stirred overnight at room temperature. The reaction was concentrated to dryness to afford 0.038 g of the title compound, a compound of the invention, as a white solid.
[0220] 1 H NMR (DMSO-d 6 )δ1.02(d, 6H), 3.57(s, 3H), 3.88(m, 1H), 7.32(s, 1H), 7.44(d, 1H), 7.62(q, 1H), 7.83(d, 1H ), 8.15 (d, 1H), 8.25 (brs, NH), 8.45 (d, 1H), 10.55 (brs, NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com