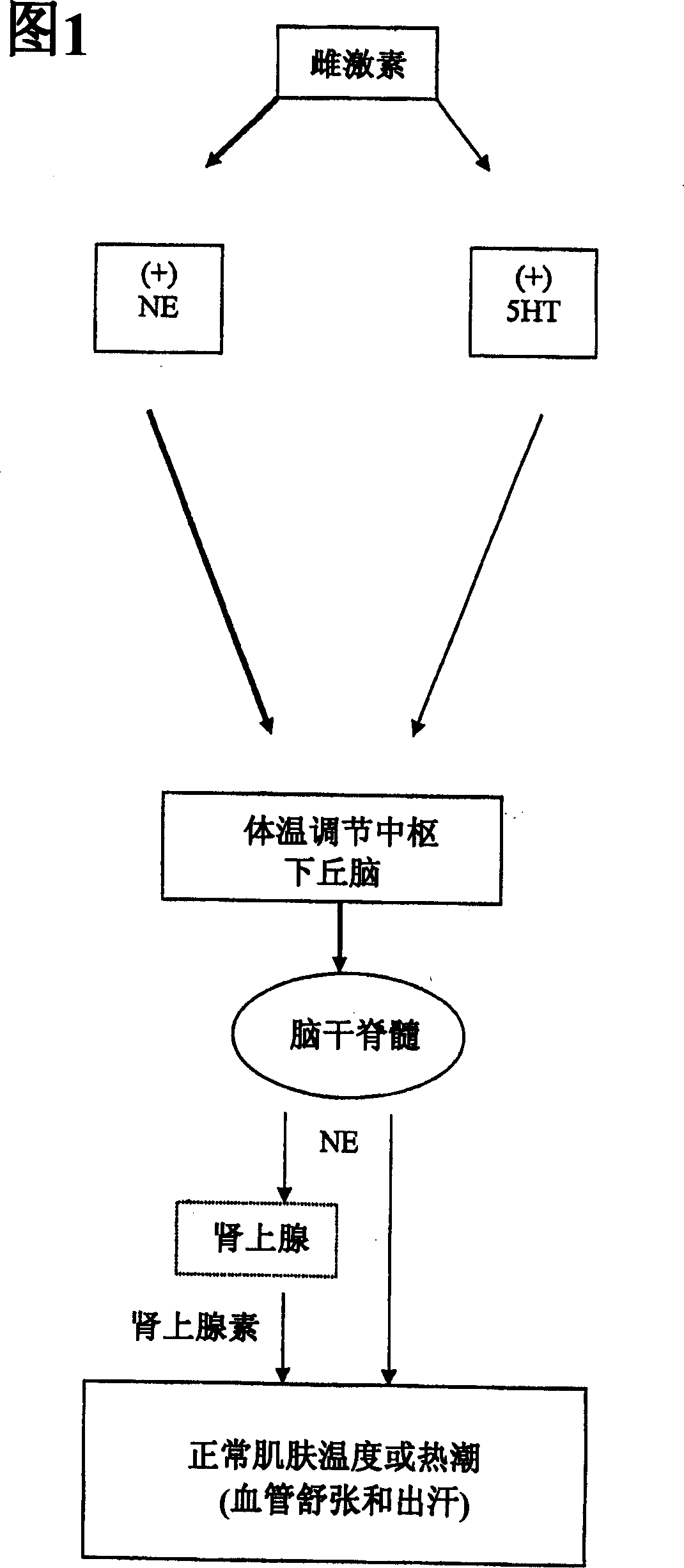
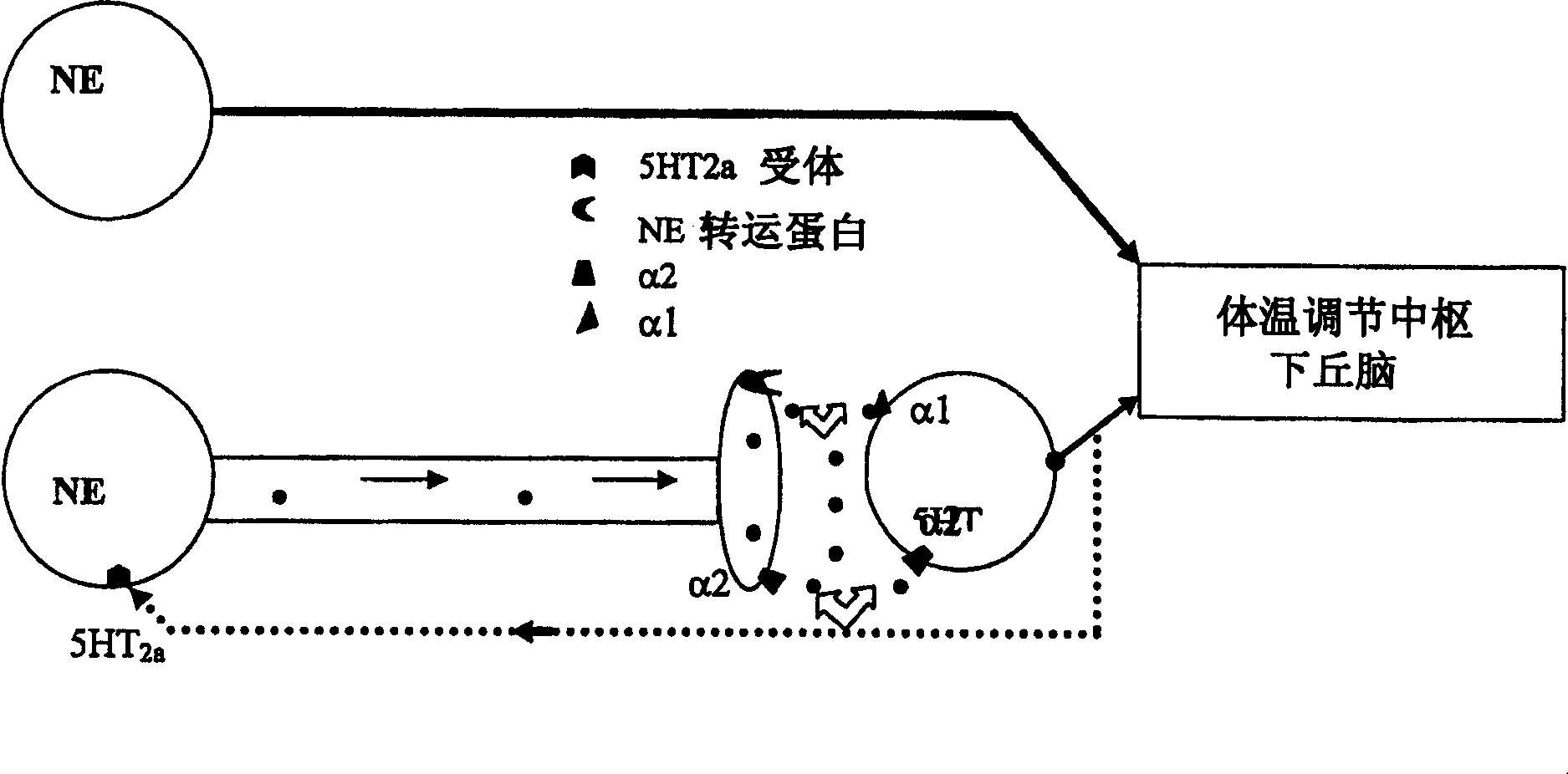
Use of norepinephrine reuptake modulators for preventing and treating vasomotor symptoms
A technology of norepinephrine, epinephrine, used in the field of prevention and treatment of vasomotor disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] In preclinical models of vasomotor instability, NRIs are effective in attenuating vasomotor instability Fixed effect
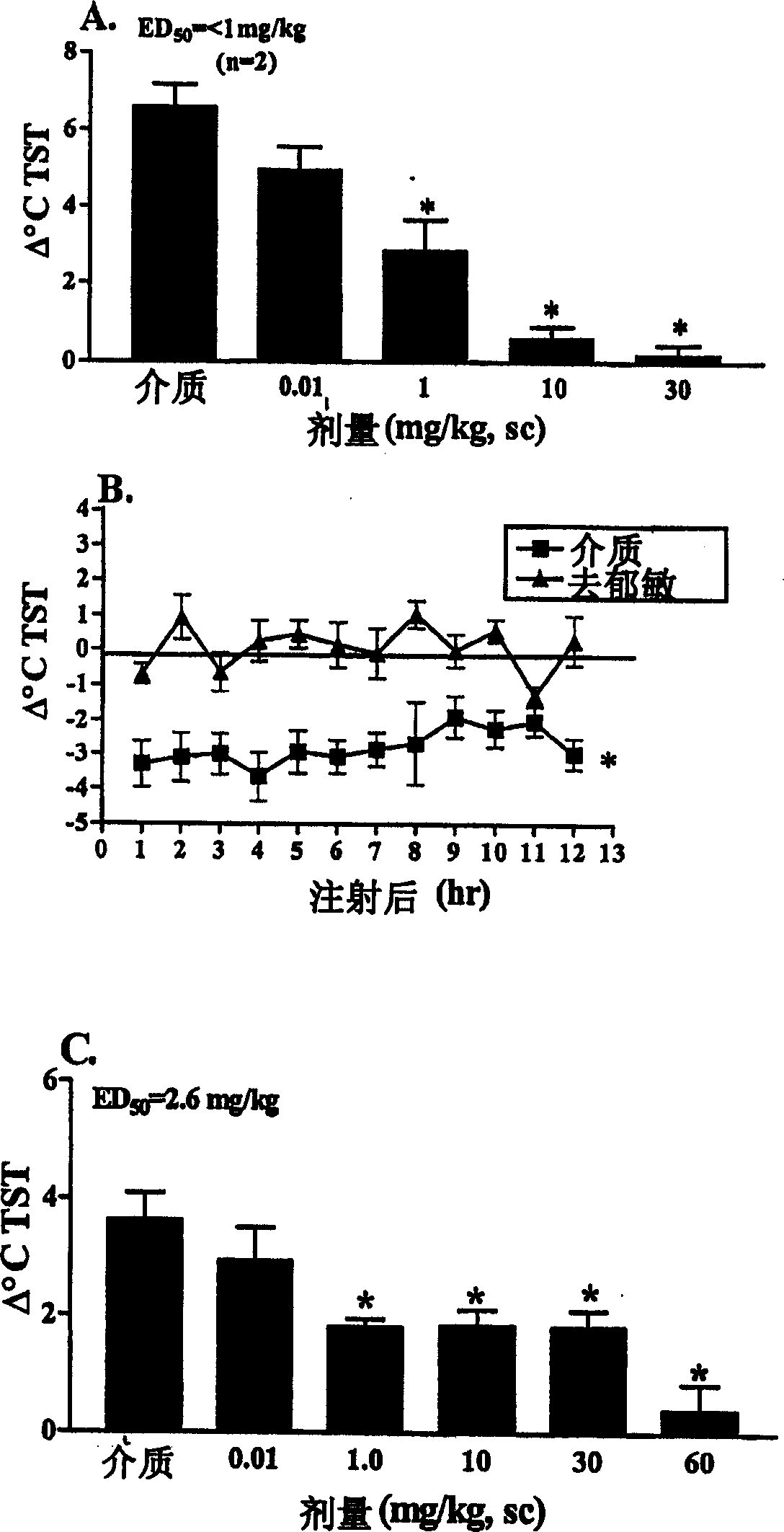
[0136] The method described in the morphine-dependent rat model in the General Methods section was used except that the rats were injected subcutaneously with vehicle (sterile water) or desipramine 1 hour before administration of naloxone. ), the desipramine can be prepared as described in US Patent Publication No. 2002 / 0107249, dissolved in sterile water and administered at 0.1, 1.0, 10 and 30 mg / kg ( image 3 A). Desipramine dose-dependently attenuated naloxone-induced hot flashes at the site of maximal hot flashes (15 minutes after naloxone administration; Δ°C, mean + SEM).
[0137] Subcutaneous injection of vehicle (sterile water) or desipramine (dissolved in sterile water and administered at 10 mg / kg) to rats ( image 3 B). In a telemetry model of OVX-induced thermoregulatory disturbances, changes in TST over time (Δ°C, mean + SEM) demonstra...
Embodiment 2
[0142] Alleviating effect of NRI combined with SRI on vasomotor instability
[0143]The method described in the morphine-dependent rat model in the General Methods section was used except that the rats were injected subcutaneously with vehicle (sterile water), desipramine 1 hour before administration of naloxone ) (prepared as described in US-A-3,454,554, dissolved in sterile water and administered at 0.1, 1.0, 10 mg / kg) or fluoxetine (fluoxetine) (Sigma, dissolved in sterile water, administered at 10, 30 , 60 mg / kg administration), or a combination of fluoxetine at 10 mg / kg and desipramine in increasing doses as listed above.
[0144] At the point of maximum hot flashes (15 minutes after naloxone administration; Δ°C, mean + SEM), desipramine dose-dependently attenuated naloxone-induced hot flashes in the MD model, but caused A slight slope of the evaluation line (solid line, Figure 4 ). The slight slope of the evaluation line is characteristic of compounds with multisit...
Embodiment 3
[0146] Alleviation of vasomotor instability by compounds with dual NRI / SRI activity
[0147] The method described in the morphine-dependent rat model in the General Methods section was adopted, except that the rats were subcutaneously injected with vehicle (sterile water), venlafaxine ( venlafaxine) (dissolved in sterile water and administered at 1.0, 10, 20, 40 mg / kg) or DVS-233 (dissolved in sterile water and administered at 1.0, 10, 30, 60 mg / kg). Venlafaxine and DVS-233 were synthesized as described in US-A-4,535,186. At the point of maximum hot flash (15 minutes after administration of naloxone; Δ°C, mean + SEM), venlafaxine dose-dependently (ED 50 Value = 15+7mg / kg) alleviated naloxone (naloxone)-induced hot flashes ( Figure 5 A). DVS-233 dose-dependently (ED 50 Value = 30+3mg / kg) alleviated naloxone (naloxone)-induced hot flashes ( Figure 5 B).
[0148] Figure 5 Methods for C and 5D were as described within the telemetry model in the general methods section....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com