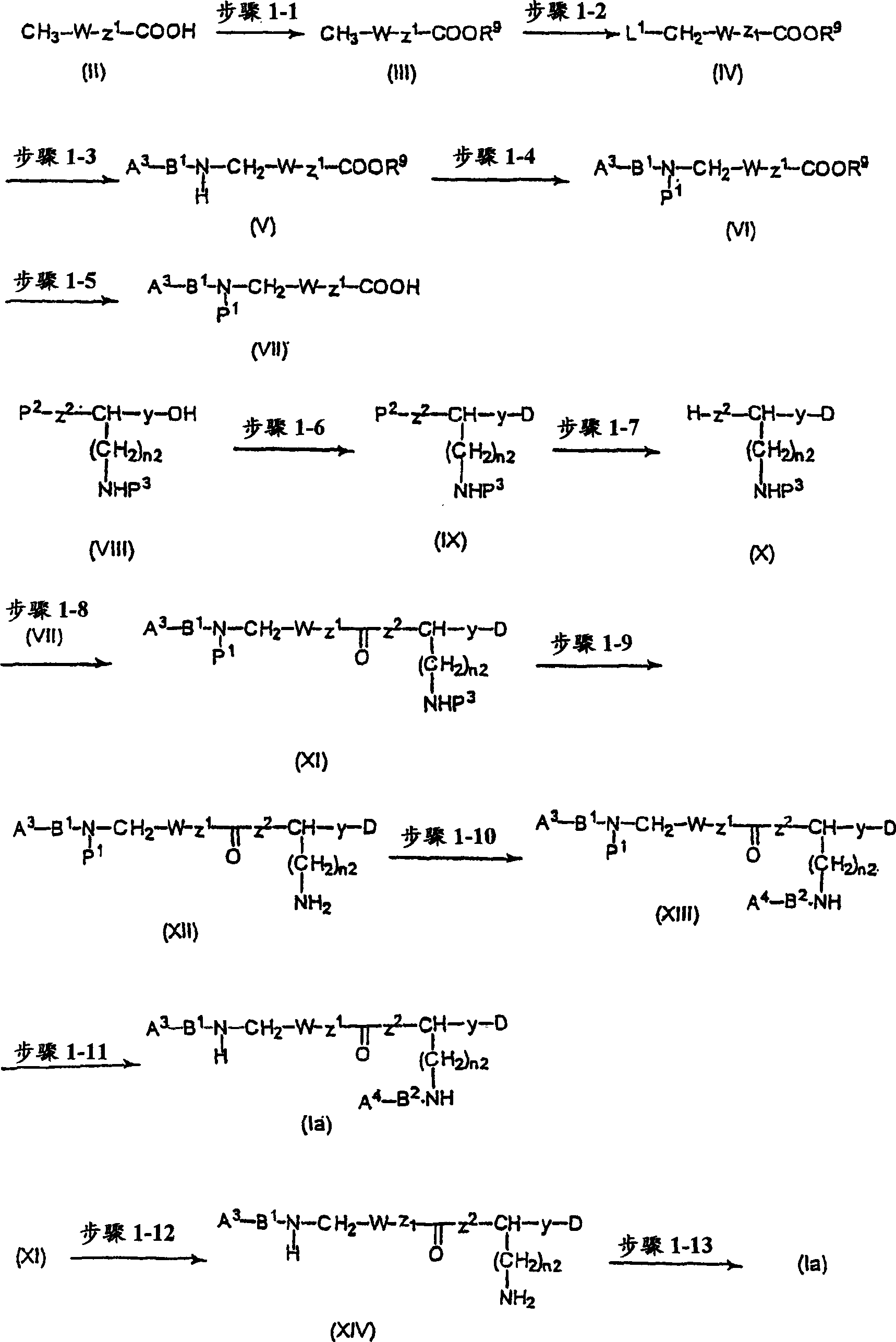
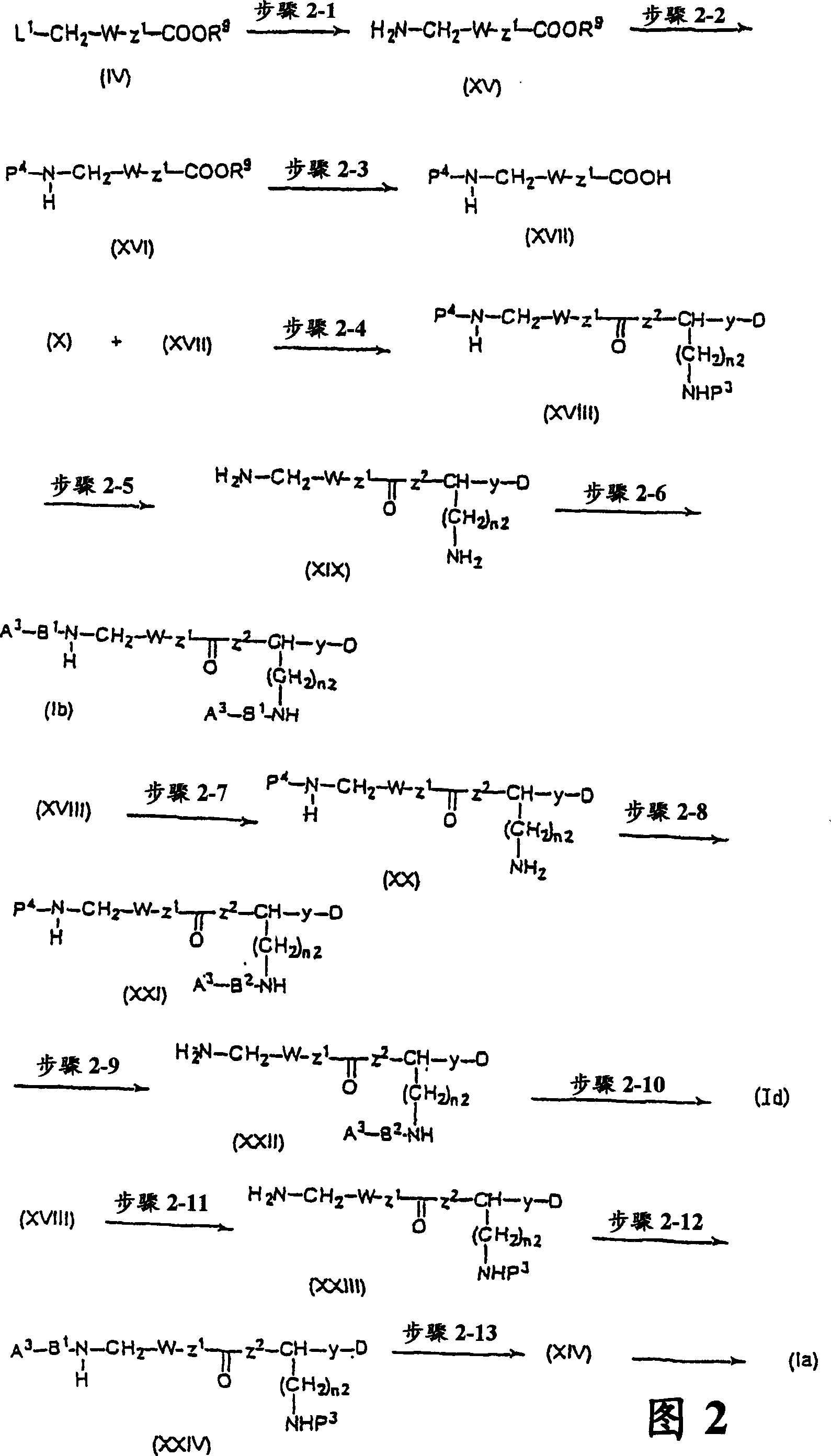
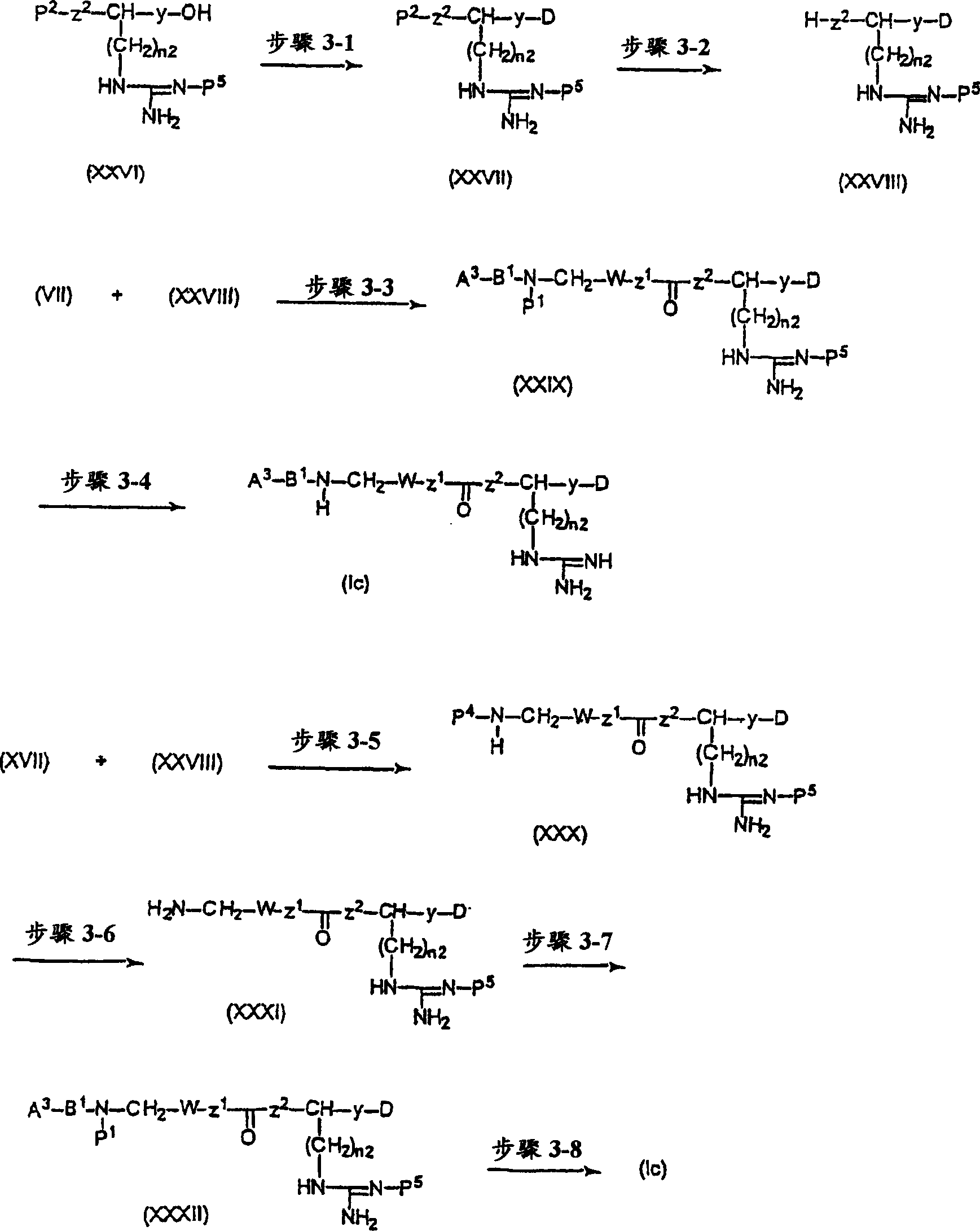
CXCR4-antagonistic drugs composed of nitrogen-containing compound
A technology of nitrogen compounds and compounds, applied in the direction of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, antineoplastic drugs, etc., can solve problems such as insufficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0327] Synthesis Example 1: (S)-2-(4-(N-2-pyridylaminomethyl)benzamido)-5-((imidazol-2-ylmethyl)amino)pentanoic acid 1-naphthalene Preparation of Methylamide [Compound No. 1]
Synthetic example 1-1
[0328] Synthesis Example 1-1: Preparation of methyl 4-(N-Boc-N-2-pyridylaminomethyl)benzoate (compound VI-1)
[0329] Dissolve 1.08 g of commercially available 2-picolylamine in 22.5 ml of DMF, add 1.55 ml of triethylamine, and cool to 0°C. To this solution was added dropwise a solution of 2.52 ml of di-tert-butyl dicarbonate dissolved in 7.5 ml of DMF over 10 minutes. After raising the temperature to room temperature and stirring for 2 hours, the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography (30 g, chloroform) to obtain 1.71 g of a pale yellow liquid.
[0330] 1.199 g of them were dissolved in 6 ml of THF, and suspended with 46.1 mg of sodium hydride (60% paraffin mixture). After stirring at room temperature for 15 minutes, 241 mg of commercially available methyl 4-bromomethylbenzoate was added, followed by stirring at room temperature for 2 days. After the reaction, adjust the pH to 5-7 with 1 mol / l hydrochl...
Synthetic example 1-2
[0333] Synthesis Example 1-2: Synthesis of 4-(N-Boc-N-2-pyridylaminomethyl)benzoic acid (Compound VII-1)
[0334] To 200.6 mg of the compound obtained in Synthesis Example 1-1 were added 2 ml of methanol, 2 ml of THF, and 2 ml of 1 mol / l sodium hydroxide aqueous solution, and stirred at room temperature for 1 day. After the reaction was completed, the solvent was distilled off, and 5 ml of water was added. 1 mol / l hydrochloric acid aqueous solution was added dropwise thereto to make pH=3. The precipitated crystals were filtered and dried to obtain 123.2 mg of the title compound as colorless crystals.
[0335] MS (Fab, pos.): m / z = 343 [M+1] +
[0336] 1 H-NMR (500MHz, DMSO-d 6): δ=1.35 and 1.54 (9H, brs), 4.41 (1H, brs), 4.51 (2H, s), 4.58 (1H, brs), 7.2-7.4 (4H, m), 7.77 (1H, td, J =7.6, 1.8Hz), 8.52(1H, dd, J=4.9, 1.7Hz), 12.9(1H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com