Recombined rhabdovirus containing double valence insect resisting gene
A technology of recombinant baculovirus and gene, applied in agricultural and forestry pest control applications, in the field of pesticides, can solve problems such as single action, insects are prone to drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
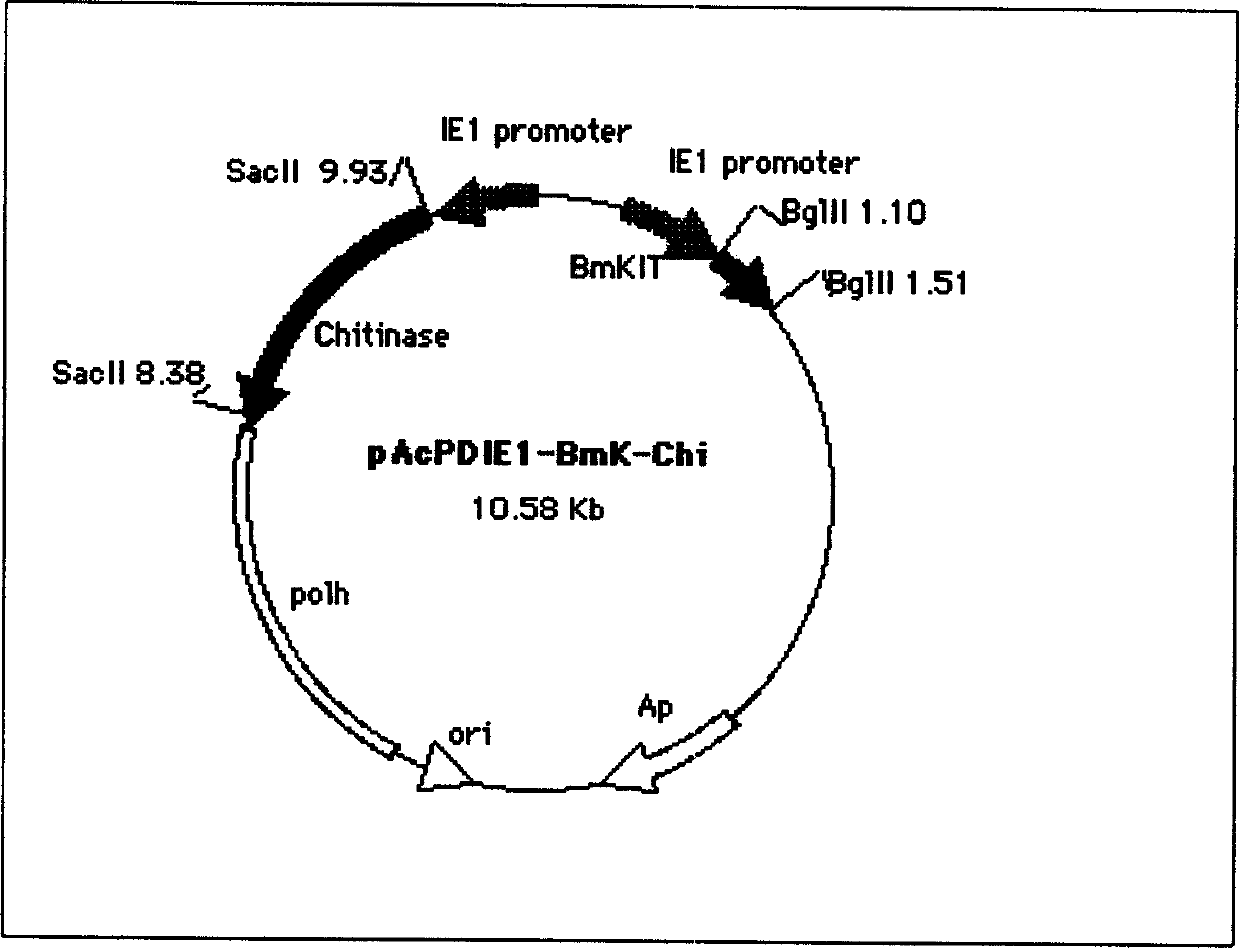
[0040] Example 1. Construction of the intermediate vector pAP DIE1-BmkIT-Chi containing bivalent insect-resistant genes
[0041] The scorpion insect toxin gene fragment and the chitinase gene fragment were respectively amplified from the plasmids pExSeP1-BmkIT and pBI101-Phitinase by CR method. Both recombinant plasmids were constructed by our laboratory (see the previous specification). The primers used to amplify the scorpion toxin gene are (BglII-sP-F: 5'-CGCG AG ATCT ATG AAA TTT TTCCTT ATA-3'; BglII-sP-FR:5'-CGCG AGA ATCT TTA ACC AAT TAT TTG G AC-3') where the underlined part is the BglII restriction site. The primers for amplifying the chitinase gene are (Chit-SaPII-F: 5'-CGG CCGCGG ATG CG AGCG AC A CTG GCG-3'; Chit-SaPII-R: CGG CCG CGG TTA GGGTTG TTG AC A TTC-3') where the underlined part is the SacII restriction site. After digesting the amplified scorpion venom gene fragment with BglII, connect it to pAP DIE1 digested with BglII, use the promoter sequence on...
Embodiment 2
[0042] Example 2, intermediate vector pAP DIE1-BmkIT-Chi and linear APN V DNA co-transfection
[0043] Inoculate sf9 cells in a 36mm-diameter plate with 2ml of GraPe's insect cell culture medium containing serum, grow on the wall for 30 minutes, remove the medium, wash twice with serum-free GraPe's insect cell culture medium, and then add 2ml of serum-free GraPe's insect cell culture medium GraPe's insect cell culture medium, let it stand for 30 minutes, discard the medium, and transfer the transfection mixture (containing 1ml serum-free GraPe's medium, 500ng linear APN V DNA and 500ng recombinant intermediate plasmid pAP DIE1-BmkIT-Chi, 4.5ul Tfx -20) Gently add to the above-mentioned plate containing cells, and after culturing at 25°C for 2 hours, add 1ml of GraPe's medium containing serum, and continue culturing at 25°C for 5 days. Virus particles and polyhedrons were collected for plaque purification.
Embodiment 3
[0044] Embodiment 3, the isolation of recombinant baculovirus
[0045] After the recombinant virus infected sf9 cells, observe the changes in the cells day by day. After a large number of polyhedrons appeared in the cells, the cells were collected by centrifugation at 11,500 rpm for 10 minutes, suspended in 50ml 0.5% SDS, incubated for 15 minutes, and the cells were broken and released. Polyhedron. The lysate was centrifuged again as above, and the precipitate was washed with water several times to remove SDS. Finally, the polyhedron was suspended in 1-2ml water, and its concentration was measured with a hemocytometer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com