Stem cells of the islets of langerhans and their use in treating diabetes
一种干细胞、糖尿病的技术,应用在胰腺细胞、非胚胎多能干细胞、基因治疗等方向,能够解决不确定等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
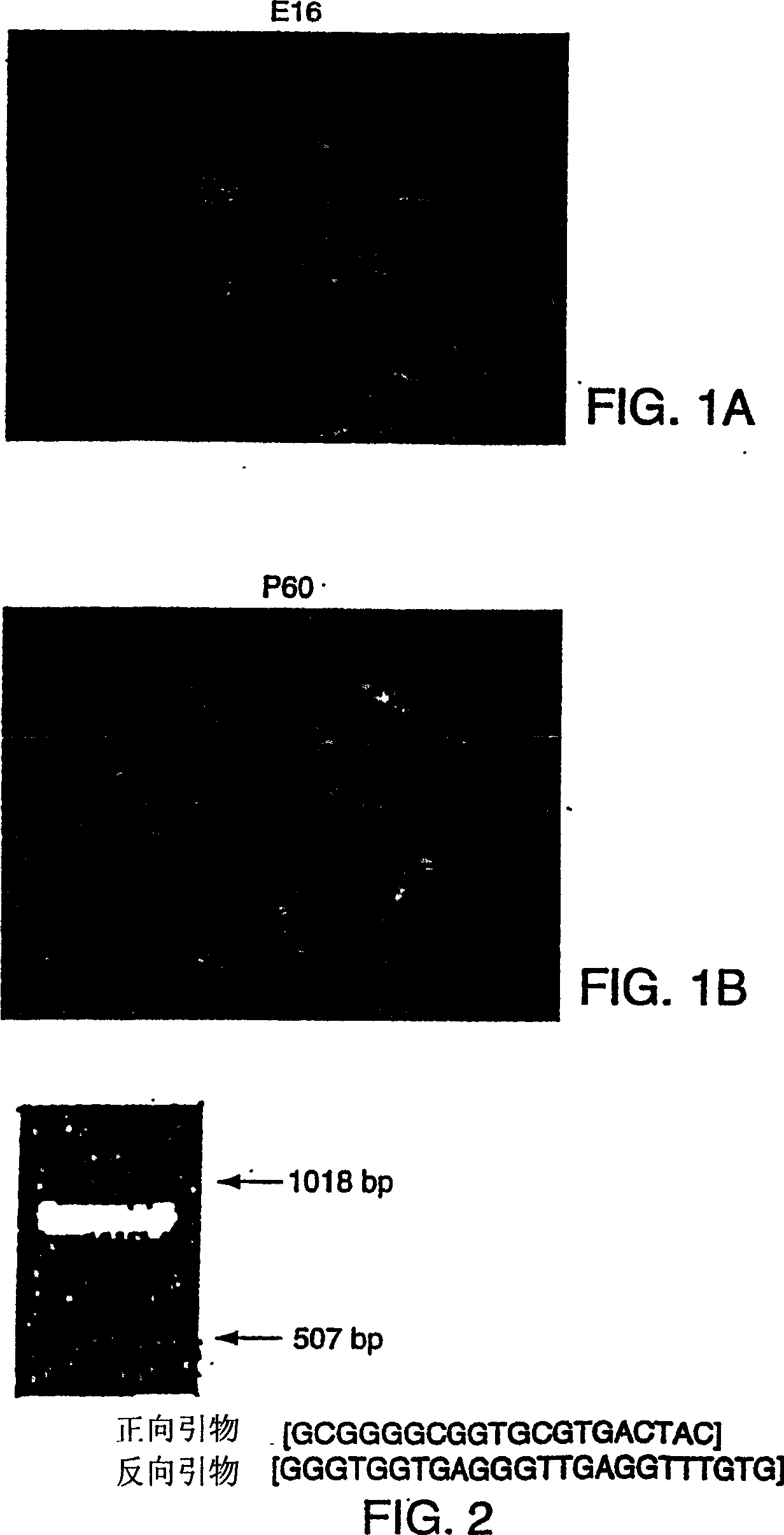
[0279] Example 1 Isolation of Nestin-Positive Stem Cells from Mouse Pancreas
[0280] Mouse islets were isolated from 2-3 month old Sprague-Dawley mice using collagenase lysis as described by Lacy and Kostianovsky. Human islets were obtained from the Diabetes Institute, Miami, FL using collagenase lysis. Islets were cultured at 37°C for 96 hours in 12-well plates (Falcon 3043 plates, Becton Dickinson, Lincoln Park, NJ) on which the plates were coated with concanavalin A. The medium was RPMI 1640 supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 10 mM HEPES buffer, 100 μg / ml streptomycin, 100 units / ml penicillin, 0.25 μg / ml amphotericin B (GIBCO BRL, Life Science Technology , Gaithersburg, MD), and 71.5 mM beta-mercaptoethanol (Sigma, St. Louis, MO).
[0281] After 96 hours, fibroblasts and other non-islet cells adhered to the surface of the concanavalin A-coated culture wells while islets were floating (not attached to the surface). At this point, the medium...
Embodiment 2
[0283] Example 2 Differentiation of pancreatic stem cells to form islets
[0284] Mouse islets were first cultured on concanavalin A-coated 12-well plates in RPMI medium containing 10% fetal bovine serum, and the islets were cultured for three days without adding culture factors except fetal bovine serum. After this period, in which the islets did not adhere, the islets were transferred to a new plate without concanavalin A.
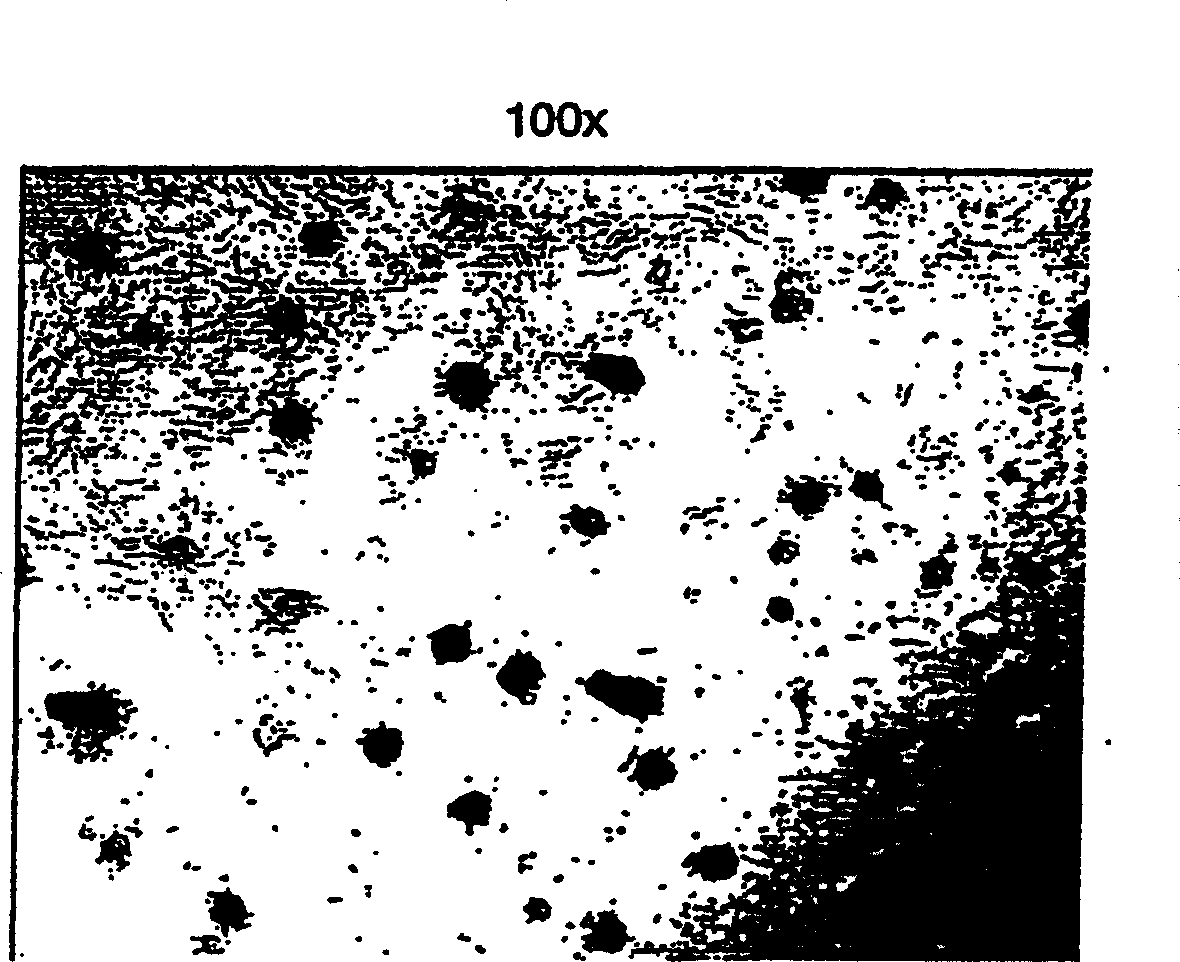
[0285] Stem cells were then stimulated to proliferate to form a monolayer from the stem cells by exposing them to bFGF-2 (20 ng / ml) and EGF (20 ng / ml) for 24 days. After 24 days, the monolayer reached abundance and surrounding the islets was a population of cells that were picked and subcloned into new 12-well plates and cultured again in medium containing bFGF and EGF.
[0286] Subclones rapidly asexually multiply into the monolayer and expand outward from the center. Cells reached abundance by day 6 and overlapped cell waves by day 12. On the 17th...
Embodiment 3
[0287] Example 3 Isolation and culture of human or mouse islets
[0288] Isolation and culture of human islets. Human islet tissue was obtained from the Islet Allocation Program of the Cell Transplantation Center, Diabetes Institute, University of Miami School of Medicine, Harvard Medical School, Boston, MA, Juvenile Diabetes Foundation for Islet Transplantation. Thoroughly washed islets were hand-picked , in modified RPMI 1640 medium (11.1 mM glucose) supplemented with 10% fetal bovine serum, 10 mM HEPES buffer, 1 mM sodium pyruvate, 100 U per mL penicillin G sodium salt, 100 μg / mL streptomycin sulfate, 0.25 ng / mL Amphotericin B, and 71.5uM β-mercaptoethanol, and Eagle 3043 added to a 12-well tissue culture plate coated with Concanavalin A (ConA). Islets were cultured at 37°C for 96 hours in the presence of 5% air and 5% CO2. Under these conditions, more islets remain in suspension (floating), while fibroblasts and other non-islet cells adhere to the medium, and the medium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com