Oxalate for preparing ambrox or releasing ambrox in cigarette smoke and its use
A technology for ambrox and cigarette smoke, which is applied in the fields of application, tobacco, and tobacco treatment, etc. It can solve the problems that the thermal decomposition efficiency is not as good as the thermal decomposition efficiency of oxalate, and the release efficiency is not very effective.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl)ethanol oxalate diester
[0027] Dissolve 0.02mol (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl)ethanol in 30ml THF, add 3.2ml pyridine, cool with ice water, add dropwise 0.04mol oxalyl chloride while stirring. It was then stirred at room temperature for 24 hours. Add 10mL of ethanol, stir for 30 minutes, add crushed ice and 40mL 2NHCl, extract with ether (200mL×3), combine the ether layers, wash with 2NHCl, water and NaHCO 3 The aqueous solution was washed separately, dried with sodium sulfate, and the solvent was removed under reduced pressure to obtain white crystals, which were filtered by suction and dried in vacuum to obtain the oxalic acid of (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl)ethanol Diester, about 80% yield.
[0028] H NMR spectrum 1 HNMR (400MHz, CDCl 3 ): δ0.788(s, 12H, 4CH 3 ), 0.874(s, 6H, 2CH 3 ), 1.191(s, 6H, 2CH 3 ), 0.899-1.692 (m, 26H), 1.876-1.924 (m, 2H), 3.415-3.806 (m, 4H), 3.18-4.1...
Embodiment 2
[0032] Preparation of (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl) ethyl oxalate
[0033]Add 0.02mol (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl)ethanol, add 0.1g of sodium methoxide and 50ml of diethyl oxalate, and distill off the generated ethanol at 80°C under reduced pressure. After the reaction was completed, (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl)ethanol ethyl oxalate was separated by column chromatography. Mass spectral data m / z (abundance): 354 (M+, <1), 236 (2), 221 (100), 195 (20), 177 (18), 151 (90), 123 (25), 95 ( 60), 69(40). The substance was confirmed to be ethyl (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl)ethanol oxalate by analyzing its primary mass spectrum data.
Embodiment 3
[0035] The thermal analysis experiment of the oxalic acid diester of (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl)ethanol shows that the low volatility of these compounds is extremely easy to completely decompose when heated chemical properties.
[0036] Thermal analysis experiment of oxalic acid diester of (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl)ethanol
[0037] Accurately weigh about 10 mg of oxalic acid diester of (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl)ethanol, and measure TG and DSC curves on a NETZSH-STA 449C thermal analyzer.
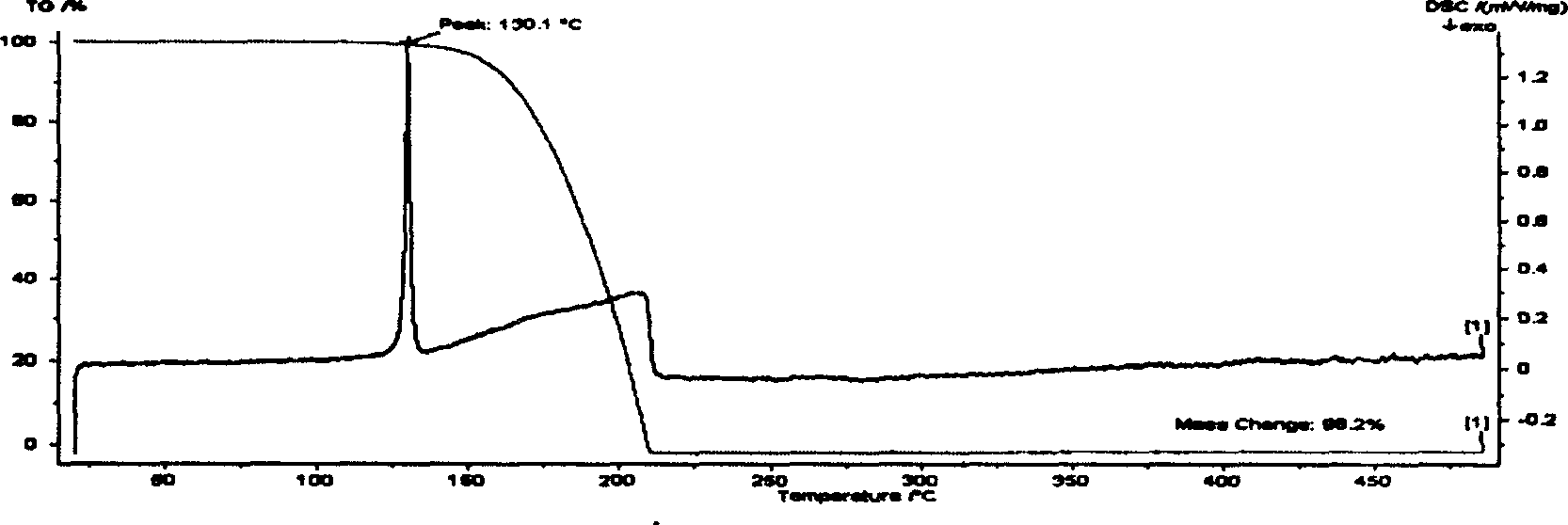
[0038] The thermal analysis curve of the oxalic acid diester of (2-hydroxy-2,5,5,8a-tetramethyl-decalinyl)ethanol is shown in figure 1 . Its melting point is measured to be 129.0-131.4°C, indicating that its volatility is low. This compound decomposes completely between 162-211°C, with a weight loss rate of 98.2%, indicating that it is almost completely decomposed when heated, and can be converted into other components very effectively when h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com