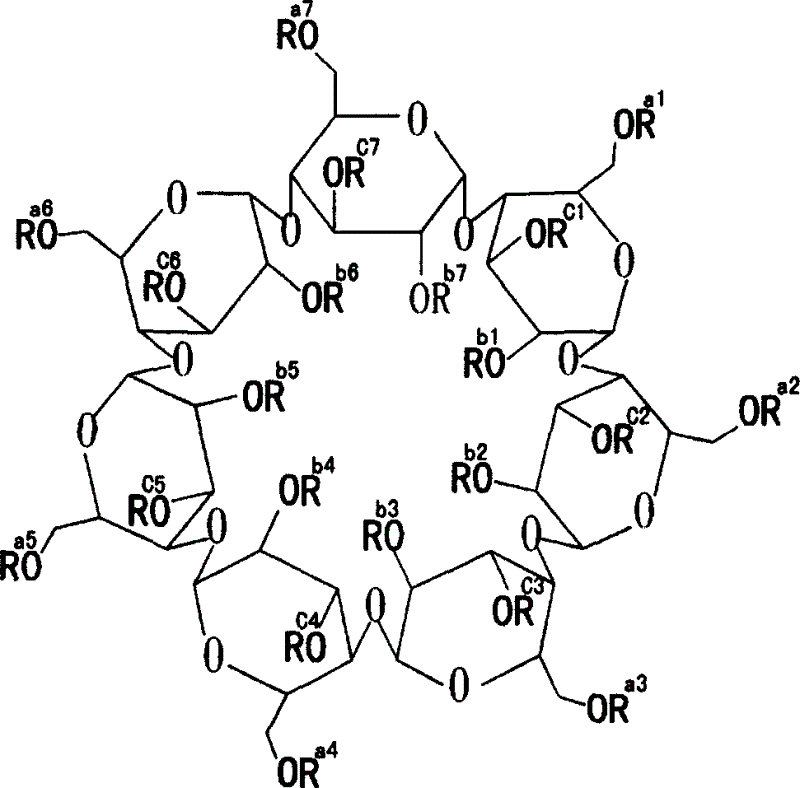
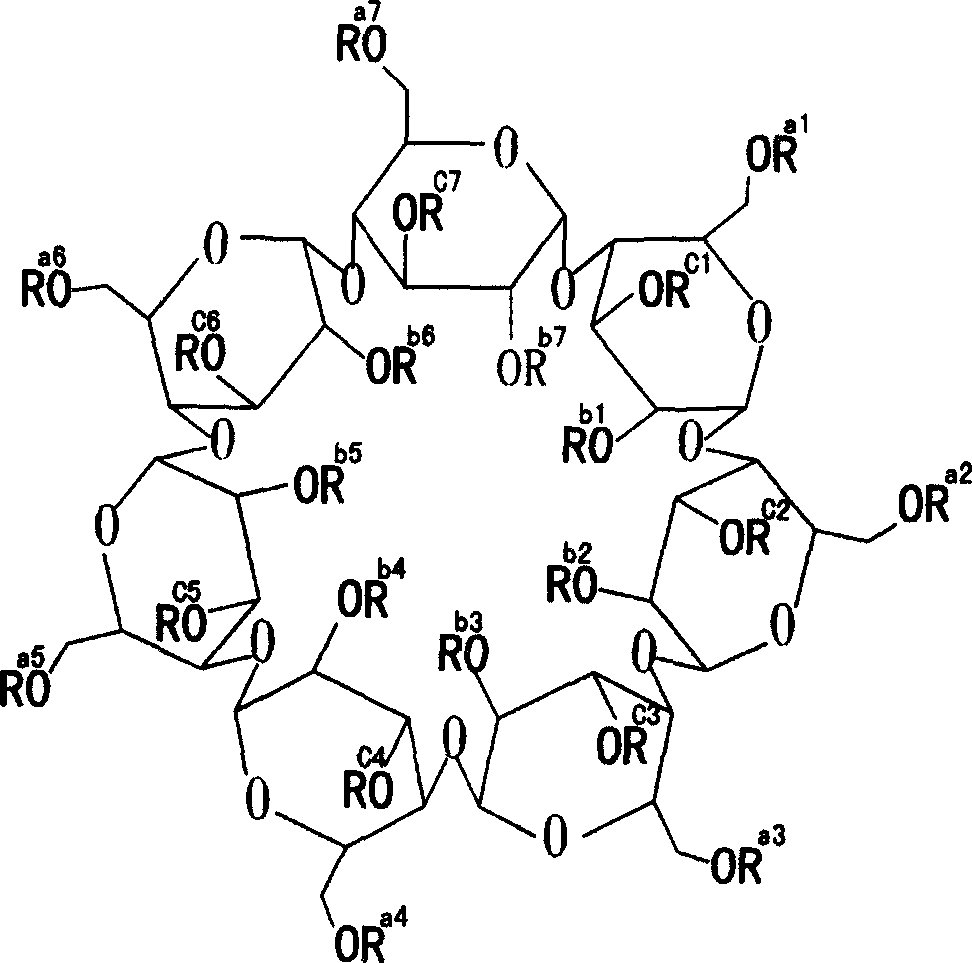
Medicinal preparation containing erythromycine ethylsuccinate
A technology of erythromycin ethylsuccinate and pharmaceutical preparations, applied in the directions of medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulations, etc., can solve the problem of successful development of parenteral administration formulations of erythromycin ethylsuccinate. and other problems, to achieve the effect of rapid curative effect, stable performance and low side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Component Specification mg
[0020] erythromycin ethylsuccinate Lijun Group 10
[0021] Hydroxypropyl Beta Cyclodextrin Shaanxi Institute of Microbiology 500
[0022] Glucose Chinese Pharmacopoeia 2000 490
[0023] Total 1000
[0024] Add hydroxypropyl beta-cyclodextrin to 5ml of water under stirring conditions, and continue to stir until it is completely dissolved; add ethylsuccinate and stir until it is completely dissolved; microwave treatment in an ice bath for 2 minutes; the resulting solution is sterilized with 0.2 mm nylon membrane filter; add medicinal glucose powder, stir until dissolved; after freeze-drying, it can be packaged and used.
Embodiment 2
[0026] Component Specification mg
[0027] erythromycin ethylsuccinate Lijun Group 50
[0028] Hydroxypropyl Beta Cyclodextrin Shaanxi Institute of Microbiology 500
[0029] Total 550
[0030] Add hydroxypropyl beta-cyclodextrin to 5ml of medicinal absolute ethanol under stirring conditions, and continue to stir until completely dissolved; add ethylsuccinate and stir until completely dissolved; the resulting solution is sterilized by filter membrane; After being freeze-dried, it can be packaged and used.
Embodiment 3
[0032] Component Specification mg
[0033] erythromycin ethylsuccinate Lijun Group 100
[0034] Hydroxypropyl Beta Cyclodextrin Shaanxi Institute of Microbiology 500
[0035] Total 600
[0036] Add hydroxypropyl beta-cyclodextrin to 5ml of medicinal absolute ethanol under stirring conditions, and continue to stir until completely dissolved; add ethylsuccinate and stir until completely dissolved; ultrasonically treat in an ice bath for 10 minutes; the resulting solution Filtered through a sterilized 0.2mm nylon membrane; after being freeze-dried, it can be packaged for use.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap