Growth factor complex
A growth factor, protein complex technology, applied in the field of regulating cell proliferation and/or migration, growth factor complex, can solve the problem of low IGF affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
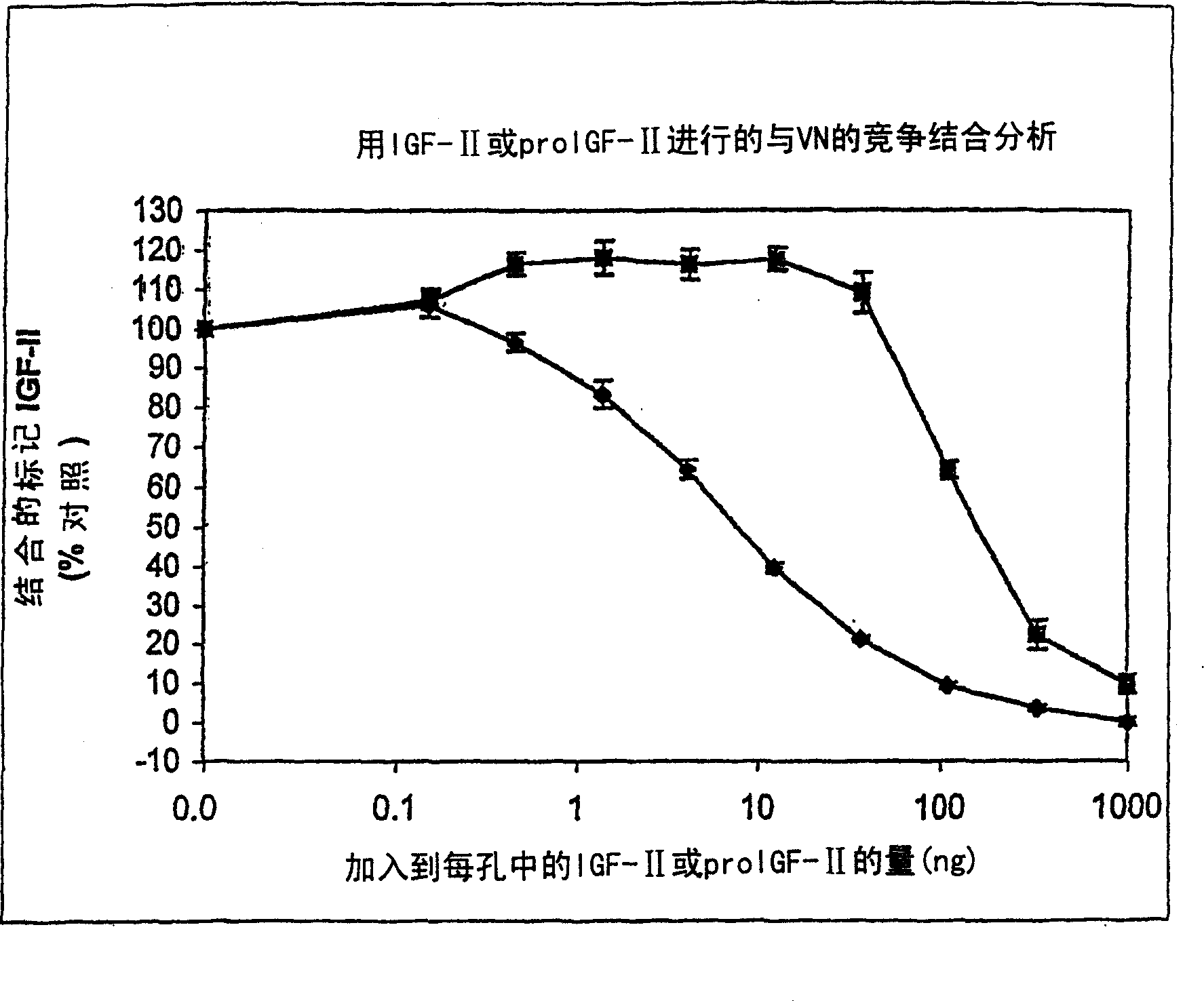
[0179] Example 1: Competition binding assay to determine the ability of insulin, pro-IGF-II and IGF-I to compete with IGF-II for vitronectin binding
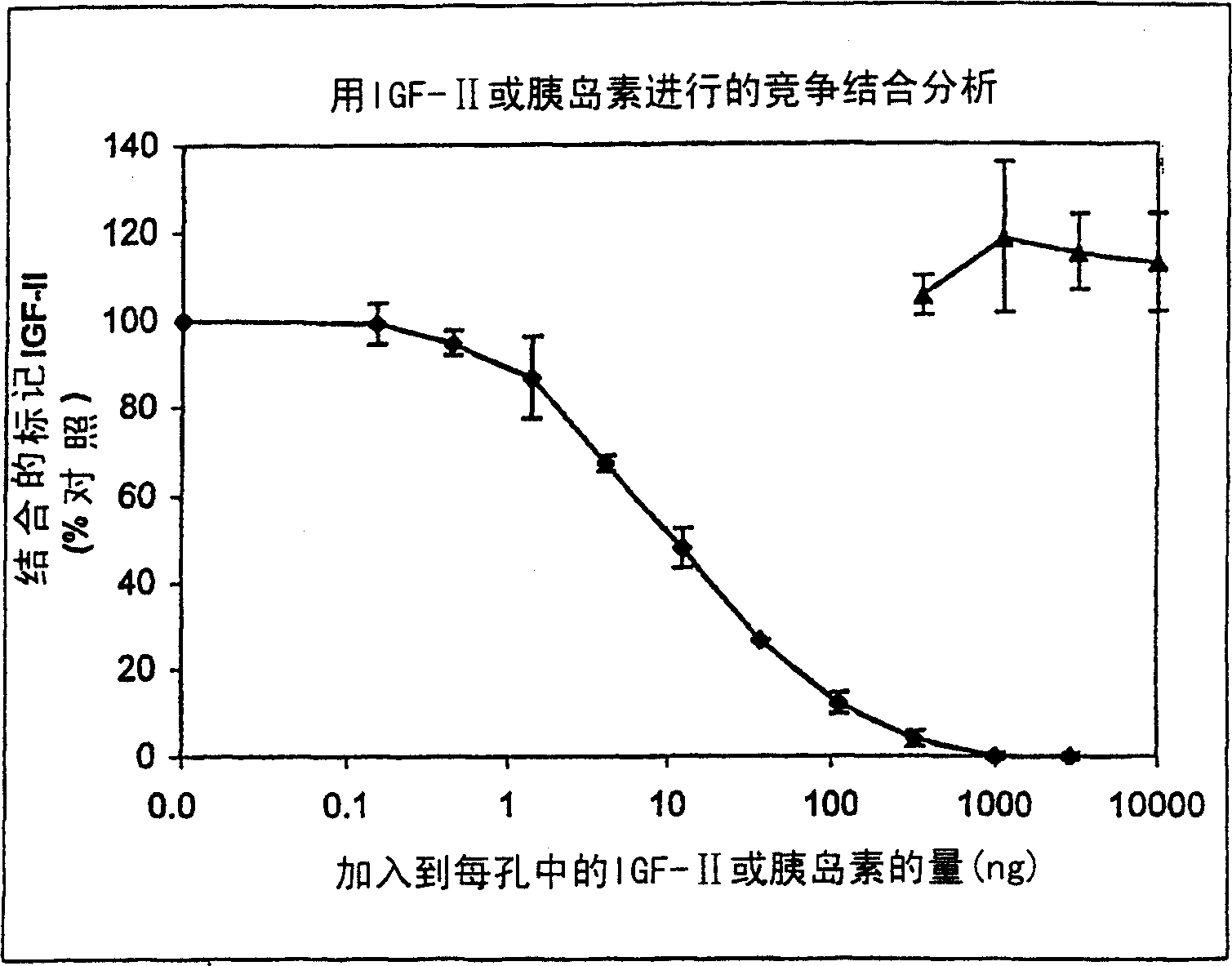
[0180] Due to the structural similarity between IGF and insulin, the binding of insulin to vitronectin was tested. Cross-linking experiments performed by Upton et al., 1999 (supra) showed that insulin is unlikely to compete with IGF-II for binding to vitronectin, as was the case with IGF-I. Therefore, high concentrations of insulin were tested to determine whether insulin could compete with radiolabeled IGF-II for vitronectin binding. shown in figure 1 The results showed that insulin was hardly related to [ 125 I]-IGF-II competes for vitronectin binding.
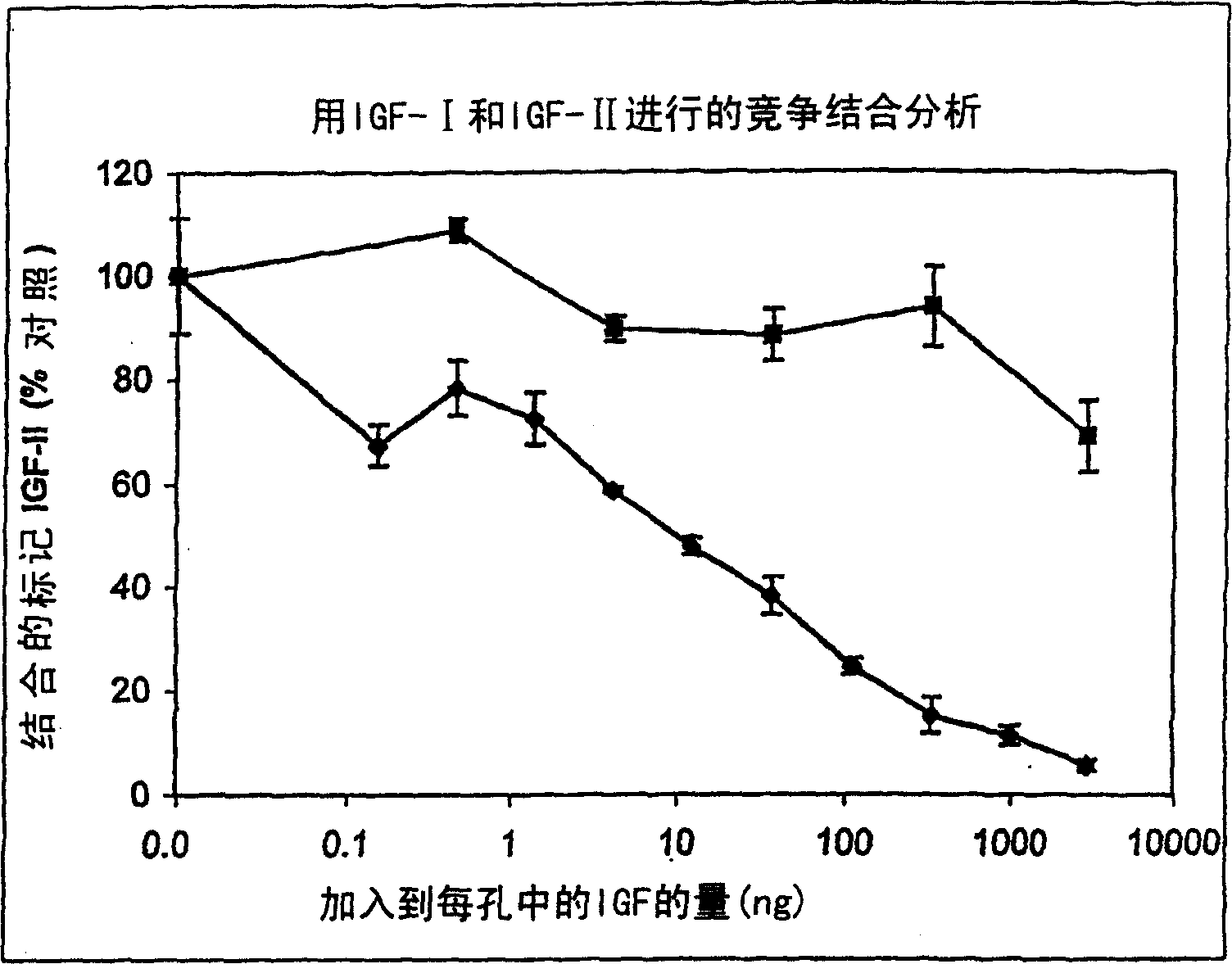
[0181] Studies of IGF-I as a competitor of IGF-II binding to vitronectin suggest that IGF-I can compete[ 125 I]-IGF-II combined with vitronectin ( figure 2 ). However, IGF-I was significantly less potent than IGF-II in competing with radiolabeled IGF-II for vitronectin ...
Embodiment 2
[0183] Example 2: Study of PAI-1 and uPAR as Competitors for IGF-II Binding to Vitronectin
[0184] PAI-1 and suPAR were investigated as possible molecules that compete with IGF-II for vitronectin binding, since these proteins have been reported to bind vitronectin (Declerck et al., 1988, J. Biol. Chem. 263 15454; Wei et al., 1994, Biol. Chem. Journal 269 32380; Kanse et al., 1996, Exp. Cell Res. 224 344). At concentrations up to 2000nM, PAI-1 was observed to interact with [ 125 I]-IGF-II competes for vitronectin IC binding 50 The value is about 524nM ( Figure 4 ). Although from IC 50This concentration appears to be relatively high, but such concentrations have been found to be relevant to tumors in vivo (Grondahl-Hansen et al., 1993, Cancer Research 53 2513). In fact, Kjoller et al., 1997, Exp. Cell Res. 232 420, found that 370 nM of PAI-1 was required to achieve a half-maximal effect in an assay determining the ability of PAI-1 to inhibit migration of WISH cells. This...
Embodiment 3
[0189] Example 3: Binding of IGF-I and IGF-II to vitronectin in the presence of non-glycosylated recombinant IGFBP-3
[0190] To investigate whether IGFBP could mediate the binding of IGF to vitronectin, increasing concentrations of IGFBP-3 in the presence of [ 125 I]-IGF-I or [ 125 I]-IGF-II in the case of the role. The results are shown in Figure 5 .
[0191] At the concentrations tested, 10 ng / well of IGFBP-3 resulted in the greatest amount of [ 125 I]-IGF-I bound to vitronectin-coated wells, while 30ng / well of IGFBP-3 resulted in the greatest amount of [ 125 I]-IGF-II binds to vitronectin-coated wells. Said role in [ 125 This is most evident in the case of I]-IGF-I, as low counts of [ 125 I]-IGF-I bound vitronectin, and even at the lowest concentration of IGFBP-3 (3.7ng), this binding was increased ( Figure 5 A). on the contrary,[ 125 I]-IGF-II did not show the increase in binding obtained on vitronectin-coated wells up to a concentration of 11 ng / 100 μL-33 ng / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com