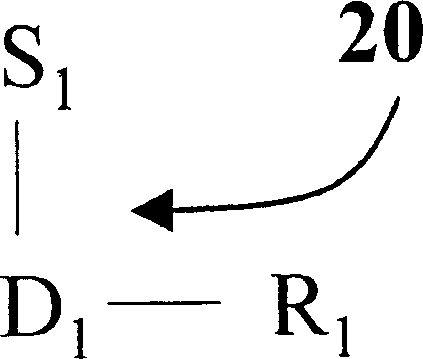Organosol liquid toner including amphipathic copolymeric binder having crosslinkable functionality
An amphiphilic copolymer, organosol technology, applied in the development agent, electrography, optics, etc., can solve the problems of adding extra process steps to apply a protective layer, undesired, disturbing the color stability of color composites, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
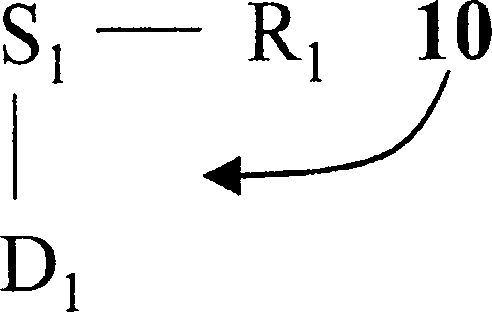
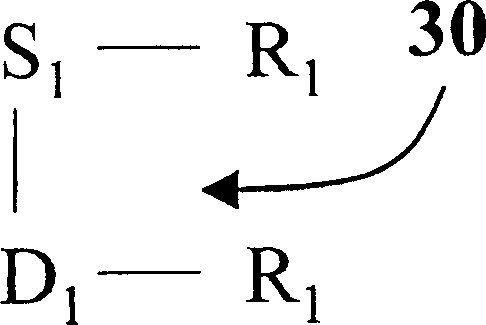
Image
Examples
Embodiment 1
[0163] Embodiment 1 (comparison)
[0164] Into a 5000 ml 3 necked round bottom flask equipped with a condenser, a thermocouple connected to a digital temperature controller, a nitrogen inlet tube connected to dry nitrogen, and a magnetic stirrer, was charged 2561 g of Norpar TM 15,849g of LMA, 26.8g of 98% HEMA and 8.75g of V601. While stirring the mixture, the reaction flask was purged with dry nitrogen at a flow rate of approximately 2 liters / minute for 30 minutes. A hollow glass stopper was then inserted into the open end of the condenser and the nitrogen flow rate was reduced to approximately 0.5 L / min. The mixture was heated to 70°C for 16 hours. Quantitative determination of conversion.
[0165] The mixture was then heated to 90°C and held at this temperature for 1 hour to destroy any remaining V601, then cooled back to 70°C. Then, the nitrogen inlet tube was removed, and 13.6 g of 95% DBTDL was added to the mixture, followed by 41.1 g of TMI. TMI was added dropwi...
Embodiment 2
[0168] Utilize the method and device of embodiment 1, combine 2561gNorpar TM 15. 823g of LMA, 26g of DAAM, 26.8g of 98% HEMA and 8.75g of V601, the resulting mixture was reacted at 70°C for 16 hours. The mixture was then heated to 90°C and held at this temperature for 1 hour to destroy any remaining V601 before cooling back to 70°C. 13.6 g of 95% DBTDL and 41.1 g of TMI were then added to the cooled mixture. TMI was added dropwise over a period of approximately 5 minutes while stirring the reaction mixture. Following the procedure of Example 1, the mixture was allowed to react at 70°C for about 6 hours, at which point the conversion was quantitative. The mixture was then cooled to room temperature. The cooled mixture was a viscous, clear solution containing invisible insoluble mater.
[0169] Using the halogen drying method described above, the liquid mixture was found to have a percent solids of 24.47%. The molecular weight was subsequently determined using the GPC metho...
Embodiment 3
[0171] Utilize the method and device of embodiment 1, combine 2561gNorpar TM 15. 823g of LMA, 26g of MAA, 26.8g of 98% HEMA and 8.75g of V601, the resulting mixture was reacted at 70°C for 16 hours. The mixture was then heated to 90°C for 1 hour to destroy any remaining V601 before cooling back to 70°C. 13.6 g of 95% DBTDL and 41.1 g of TMI were then added to the cooled mixture. TMI was added dropwise over a period of approximately 5 minutes while stirring the reaction mixture. Following the procedure of Example 1, the mixture was allowed to react at 70°C for about 6 hours, at which point the conversion was quantitative. The mixture was then cooled to room temperature. The cooled mixture was a viscous, clear solution containing invisible insoluble mater.
[0172] Using the halogen drying method described above, the liquid mixture was found to have a percent solids of 25.10%. The molecular weight was subsequently determined using the GPC method described above; based on tw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com