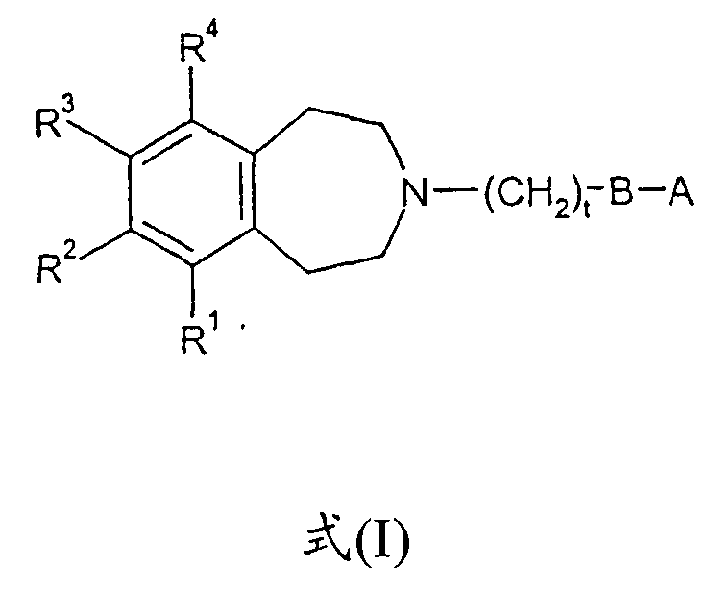
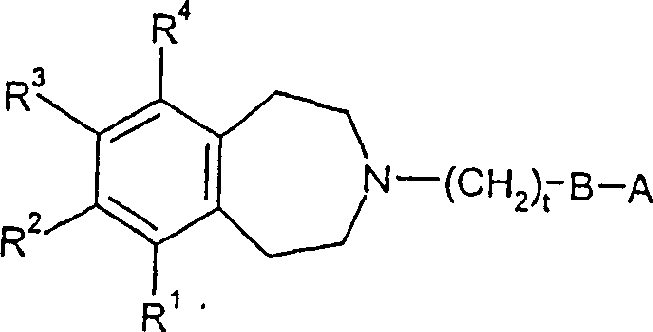
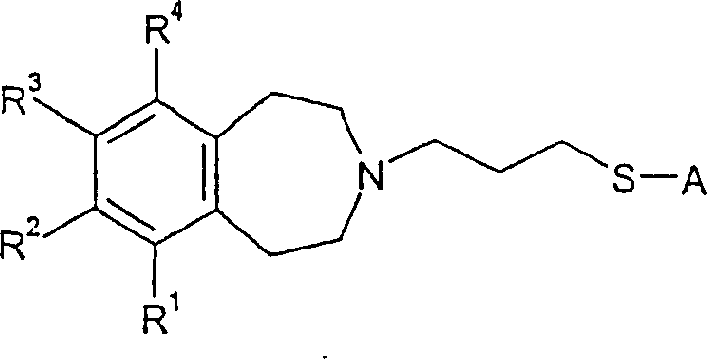
Tetrahydroazepine derivatives useful as dopamine d3 receptor modulators (antipsychotic agents)
A technology of compound and alkyl, applied in the field of novel tetrahydrobenzazepine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 173
[0506] 7-(5-methyl-isoxazol-3-yl)-3-{4-[4-methyl-5-(2-methyl-quinolin-6-yl)-4H-[1,2 ,4] Triazol-3-yl]-butyl}-2,3,4,5-tetrahydro-1H-benzo[d]azepine
[0507] a) 4-[1,3]dioxan-2-yl-butyric acid methyl ester
[0508] Methyl 5,5-dimethoxyvalerate (20.0 g, 0.114 mol), propane-1,3- Diol (13 g, 0.17 mol) and p-toluenesulfonic acid (2.17 g, 0.0114 mol) for 4 hours. The mixture was cooled to room temperature, diluted with diethyl ether (100ml), then neutralized with solid sodium bicarbonate, the solid filtered and the filtrate evaporated to give a pale yellow oil (22.3g). 10 g of this material was purified by silica gel chromatography (eluent: 30% ethyl acetate:hexane) to obtain the title compound (6.82 g) as a colorless oil.
[0509] b) 4-[1,3]dioxan-2-yl-butanoic acid
[0510] 4-[1,3]dioxan-2-yl-butyric acid methyl ester (6.8 g, 0.036 mol), the mixture was stirred at room temperature for 18 hours, then the solvent was evaporated. The resulting residue was partitioned with ethyl ...
Embodiment 174
[0529] 3-14-[5-(4-fluoro-phenyl)-4-methyl-4H-[1,2,4]triazol-3-yl]-butyl}-7-(5-methyliso Oxazol-3-yl)-2,3,4,5-tetrahydro-1H-benzo[d]azepine
[0530] Mass spectrometry (API + ): measured value 460 (MH + ). C 27 h 30 N 5 OF expects 459.
[0531]
Embodiment 175
[0533] 7-Ethylsulfonyl-3-[4-(4-methyl-5-quinolin-6-yl-4H-[1,2,4]triazol-3-ylthio)butyl]-2,3 , 4,5-Tetrahydro-1H-benzo[d]azepine
[0534] a) 6-{5-[3-(5,5-dimethyl-[1,3]dioxan-2-yl)-propylthio]-4-methyl-4H-[1,2, 4] Triazol-3-yl}-quinoline
[0535] In dimethylformamide, 4-methyl-5-quinolin-6-yl-4H-[1,2,4]triazole-3-thiol (0.5g, 2.07mmol) was heated at 100°C , 2-(3-bromopropyl)-5,5-dimethyl-[1,3]dioxane (0.49g, 2.07mmol) and lithium hydroxide (50mg) for 3 hours, the mixture was cooled and washed with water ( 80ml) and ethyl acetate (100ml). After separating the aqueous and organic layers, the aqueous layer was re-extracted with ethyl acetate (100ml). The combined organic phases were washed with brine (100ml), and washed with Na 2 SO 4 Drying, filtration and evaporation gave a colorless oily substance. Purification by silica gel chromatography (eluent: ethyl acetate-10% methanol: ethyl acetate) afforded the title compound (0.53 g, 65%) as a colorless solid.
[0536] Mass sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com