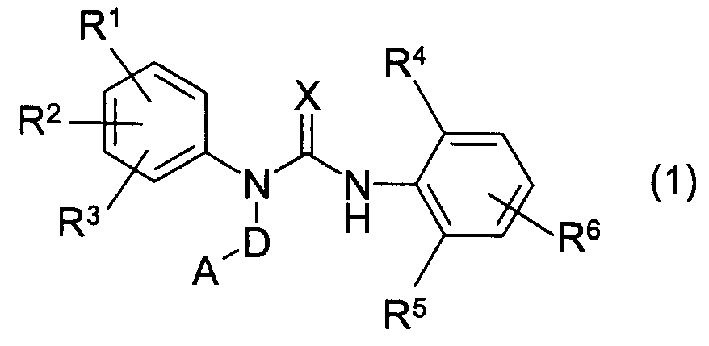
Novel 3-substituted urea derivatives and medicinal use thereof
A technology of urea derivatives and substituents, which is applied in the field of new 3-substituted urea derivatives and their pharmaceutical applications, and can solve the problems of no discovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0104] To a solution of benzaldehyde (10 mL) in toluene (200 mL) were added 4-octylaniline (22.5 mL) and molecular sieve 4A (20 g) under ice-cooling, and the mixture was stirred at room temperature overnight. Molecular sieve 4A was filtered off from the reaction mixture, and the resulting filtrate was concentrated under reduced pressure. The residue was dissolved in methanol (200 mL), and sodium borohydride (2.3 g) was added under ice-cooling. The mixture was stirred at room temperature for 5 hrs. Methanol was evaporated and water was added to the residue. The mixture was extracted with chloroform, and the organic layer was washed with saturated brine, dried and concentrated under reduced pressure to give benzyl(4-octylphenyl)amine (29 g). 1 H-NMR (CDCl 3 )δ: 0.85 (3H, t, J = 6.6Hz), 1.27-1.30 (10H, m), 1.59 (2H, t, J = 7.3Hz), 2.58 (2H, t, J = 7.3Hz), 4.29 ( 2H, s), 6.56 (2H, d, J = 8.6 Hz), 6.97 (2H, d, J = 8.6 Hz), 7.26-7.38 (5H, m). Preparation Example 2
preparation Embodiment 2
[0105] Use 4-methoxybenzaldehyde (1.0g) and 4-octylaniline (1.4g) as raw materials, react and process in the same manner as Preparation Example 1, to obtain [(4-methoxyphenyl) Methyl](4-octylphenyl)amine (2.2 g). 1 H-NMR (CDCl 3 )δ: 0.90 (3H, t, J = 6.6Hz), 1.22-1.51 (10H, m), 1.54 (2H, t, J = 7.9Hz), 2.48 (2H, t, J = 7.9Hz), 3.79 ( 3H, s), 4.22 (2H, s), 6.57 (2H, d, J=8.6Hz), 6.86 (2H, d, J=8.6Hz), 6.98 (2H, d, J=8.9Hz), 7.28 ( 2H, d, J = 8.9 Hz). Preparation Example 3
preparation Embodiment 3
[0106] Use 4-hydroxybenzaldehyde and 4-octylaniline as raw materials, react and treat in the same manner as Preparation Example 1, to obtain [(4-hydroxymethylphenyl)methyl](4-octylphenyl )amine. Preparation Example 4
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com