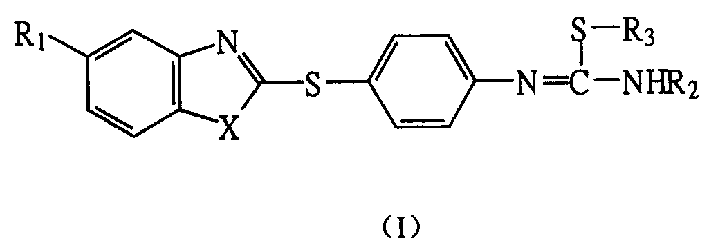
Nitrogen monoxide synthase inhibitor, its preparation method and application
A compound, methoxy technology, applied in the field of nitric oxide synthase inhibitors, can solve problems affecting application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0046] The preparation of example 1S-methyl-N-[4-(benzimidazole-2-mercapto) phenyl] isothiourea hydroiodide (I-1) Imidazole (III-1):
[0047] Add 9.5g (63.3mmol) of 2-mercaptobenzimidazole, 60ml of anhydrous acetonitrile, 2.53g (63.3mmol) of powdered sodium hydroxide, 9.98g (63.3mmol) of p-nitrochlorobenzene and 1.0g of PEG-400 into the pressure-resistant In the bottle, place it in an oil bath at 120°C under electromagnetic stirring for 28 hours, cool, filter with suction, wash twice with cold acetonitrile, and then wash with water to pH 7 to obtain 15.1 g (87.9%) of yellow solid (III-1), mp 185~ 186°C. 1 HNMR (90MHz, CDCl 3 +DMSO-d 6 ), δ(ppm): 7.17~7.32(2H, m, ArH), 7.47~7.62(4H, m, ArH), 8.04~8.20(2H, m, ArH)2-(4-aminophenylsulfanyl)benzene And imidazole (IV-1):
[0048] Add 10 g (179 mmol) of reduced iron powder and 200 ml of 95% ethanol into a three-neck flask, add 8.4 ml of 10% HCl dropwise under mechanical stirring, and after 10 min, add 1...
example 2
[0052] The preparation of example 2S-ethyl-N-[4-(benzimidazole-2-mercapto) phenyl] isothiourea hydroiodide (I-2)
[0053] Using compound (VI-1) and iodoethane (molar ratio 1:3) as raw materials, a method similar to compound (I-1) was used to obtain pale yellow crystals (I-2, 54.8%), mp 148-150°C. IR (cm -1 ): 3443, 3407(NH), 3135~2835( + NH 3 ), 3082 (ArH), 2977, 2930 (CH), 1628, 1546, 1524 (C=C, C=N), 1426, 1403 (imidazole), 1269 (-SCH 2 -), 749 (ArH, ortho-disubstituted) 1 HNMR (90MHz, DMSO-d 6 +CDCl 3 ), δ (ppm): 1.38 (3H, t, J=7.33Hz, -S-CH 2 -CH 3 ), 3.30 (2H, q, J=7.08Hz, -S-CH 2 -), 7.16~7.58 (8H, m, ArH), 7.58~9.80 (4H, br, + NH 3 +NH) MS (SCI 70eV, m / z): 329 (M+1), 312 (M-16), 284, 254, 242 (base peak) Anal (C 16 h 16 N 4 S 2 .HI; C%, H%, N%): Req 42.11, 3.75, 12.28 Found 41.89, 3.84, 12.06
example 3
[0054] The preparation of example 3S-n-butyl-N-[4-(benzimidazole-2-mercapto) phenyl] isothiourea hydroiodide (I-3)
[0055] Using compound (VI-1) and iodo-n-butane (molar ratio 1:3) as raw materials, a method similar to compound (I-1) was used to obtain light yellow crystals (I-3, 62%), mp 176-177 ℃IR(cm -1 ): 3149~2734 ( + NH 3 ), 3077 (ArH), 2958, 2928 (CH), 1627, 1587, 1544, 1516 (C=C, C=N), 1422, 1407 (imidazole), 1269 (-SCH 2 -), 737 (ArH, ortho-disubstituted) 1 HNMR (90MHz, DMSO-d 6 +CDCl 3 ), δ (ppm): 0.95 (3H, t, J=6.48Hz, -S-(CH 2 ) 3 -CH 3 ), 1.33~1.77 (4H, m, -S-CH 2 -CH 2 CH 2 -CH 3 ), 3.26 (2H, t, J=7.02Hz, -S-CH2-), 7.14~7.67 (8H, m, ArH), 7.7~10.0 (4H, br, + NH 3 +NH) MS (SCI 70eV m / z): 357 (M+1), 284, 242, 184, 57 (base peak) Anal (C 18 h 20 N 4 S 2 HI; C%, H%, N%): Req 44.63, 4.37, 11.57 Found 44.36, 4.55, 11.29
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com