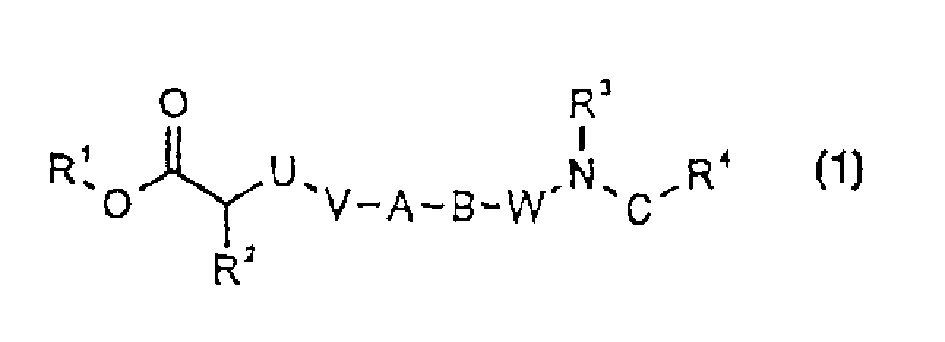Biphenyl and biphenyl-analogous compounds as integrin antagonists
A technology of compounds and residues, applied in the direction of compounds containing elements of group 3/13 of the periodic table, preparation of organic compounds, active ingredients of heterocyclic compounds, etc., can solve problems such as poorly active compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0144] Then with a solution of 564 μl diisopropylethylamine in 13 ml tetrahydrofuran (THF) / dichloromethane (1:1) and 3.13 g 4-nitrophenyl chloroformate in 13 ml tetrahydrofuran (THF) / dichloromethane The solution in (1:1) treated the resin. Shake it at room temperature for 45 minutes, wash with tetrahydrofuran (THF) and dimethylformamide (DMF), add 1.07g propylamine (amine reagent) and 3.16ml diisopropylethylamine in 23ml dimethylformamide solution in (DMF). After shaking for 2 hours, the resin was washed with dimethylformamide (DMF), methanol, tetrahydrofuran (THF) and dichloromethane. To remove the product, the resin was shaken with 10 ml of trifluoroacetic acid (TFA) / dichloromethane for 1 hour, filtered, the filtrate concentrated in vacuo and purified on silica gel to give 210 mg of the title compound. Mass spectrum (ESI): 524. Retention time (HPLC): R t = 10.4. 1 H-NMR (400MHz, methanol) δ=7.67(s, 1H), 7.32-7.22(m, 4H), 7.17(d, 1H), 7.04(d, 2H), 6.77(s, 2H), 3.93(dd ,...
Embodiment 13
[0145] Preparation of (2R, S)-3-[3'-(3-benzylureido)-biphenyl-4-yl]-2-[2,4,6-trimethylbenzenesulfonate according to the method of Example 1.1 Amino]-propionic acid, except that benzylamine was used instead of propylamine as the amine reagent. Mass spectrum (ESI): 572. Retention time (HPLC): R t = 11.0. Example 1.3: (2R,S)-3-[3'-(3-(2-pyrrolidin-1-ylethyl)-ureido)-biphenyl-4-yl]-2-[2,4 , 6-trimethylbenzenesulfonylamino]-propionic acid
Embodiment 14
[0146](2R, S)-3-[3'-(3-(2-pyrrolidin-1-ylethyl)-ureido)-biphenyl-4-yl]-2-[ 2,4,6-Trimethylbenzenesulfonylamino]-propionic acid, except that 2-pyrrolidin-1-ylethylamine was used instead of propylamine as the amine reagent. Mass spectrum (ESI): 579. Retention time (HPLC): R t = 8.3. Example 1.4: (2R,S)-3-[3'-(3-(pyridin-2-ylmethylureido)-biphenyl-4-yl]-2-[2,4,6-trimethyl phenylsulfonylamino]-propionic acid
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com