Compsns. derived from i(mycobacterium vaccae) and methods for their use
A technology of mycobacterium vaccae, composition, applied in the field of treatment, immune disorders and cancer diseases, prevention and detection including infectious diseases, treatment of respiratory diseases and skin diseases, can solve side effects, can not eradicate virus , interference with infection performance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
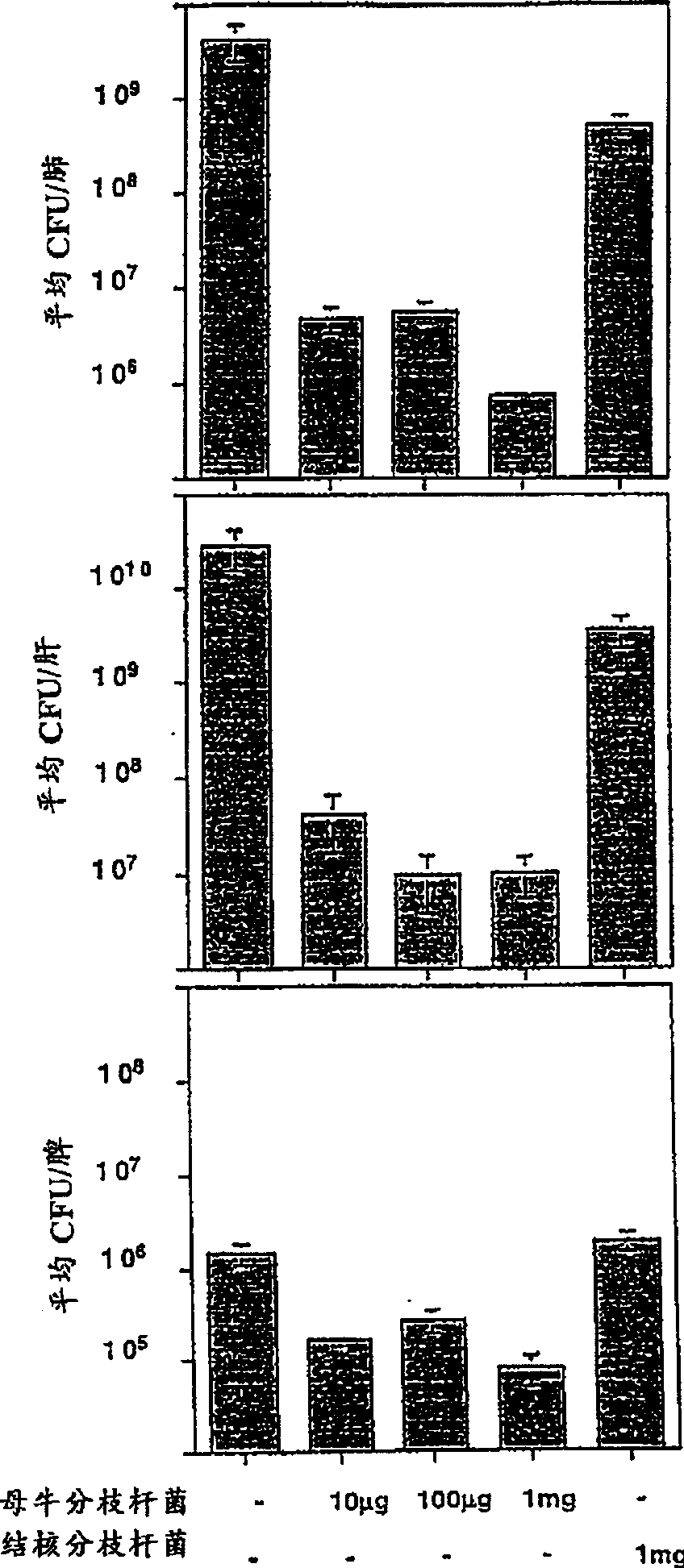
[0133] Effects of Mice Immunized with Mycobacterium vaccae on Tuberculosis
[0134] This example illustrates the effect of immunizing mice with heat-killed M. vaccae or M. vaccae culture filtrates prior to challenge with live M. tuberculosis.
[0135] Mycobacterium vaccae (ATCC No. 15483) was cultured in sterile medium 90 (yeast extract, 2.5 g / l; tryptone, 5 g / l; glucose, 1 g / l) at 37°C. Cells were harvested by centrifugation, transferred to sterile Middlebrook 7H9 medium (DifcoLaboratoies, Detroit, MI, USA) containing glucose, and cultured at 37°C for 1 day. The medium was then centrifuged to pellet the bacteria and the culture filtrate was removed. The bacterial pellet was resuspended in phosphate-buffered saline at a concentration of 10 mg / ml, equal to 10 10 Mycobacterium vaccae / ml. The bacterial suspension was then autoclaved at 120°C for 15 minutes. The culture filtrate was collected through a 0.45 μm filter into sterile bottles.
[0136] As shown in Figure 1A, when ...
Embodiment 2
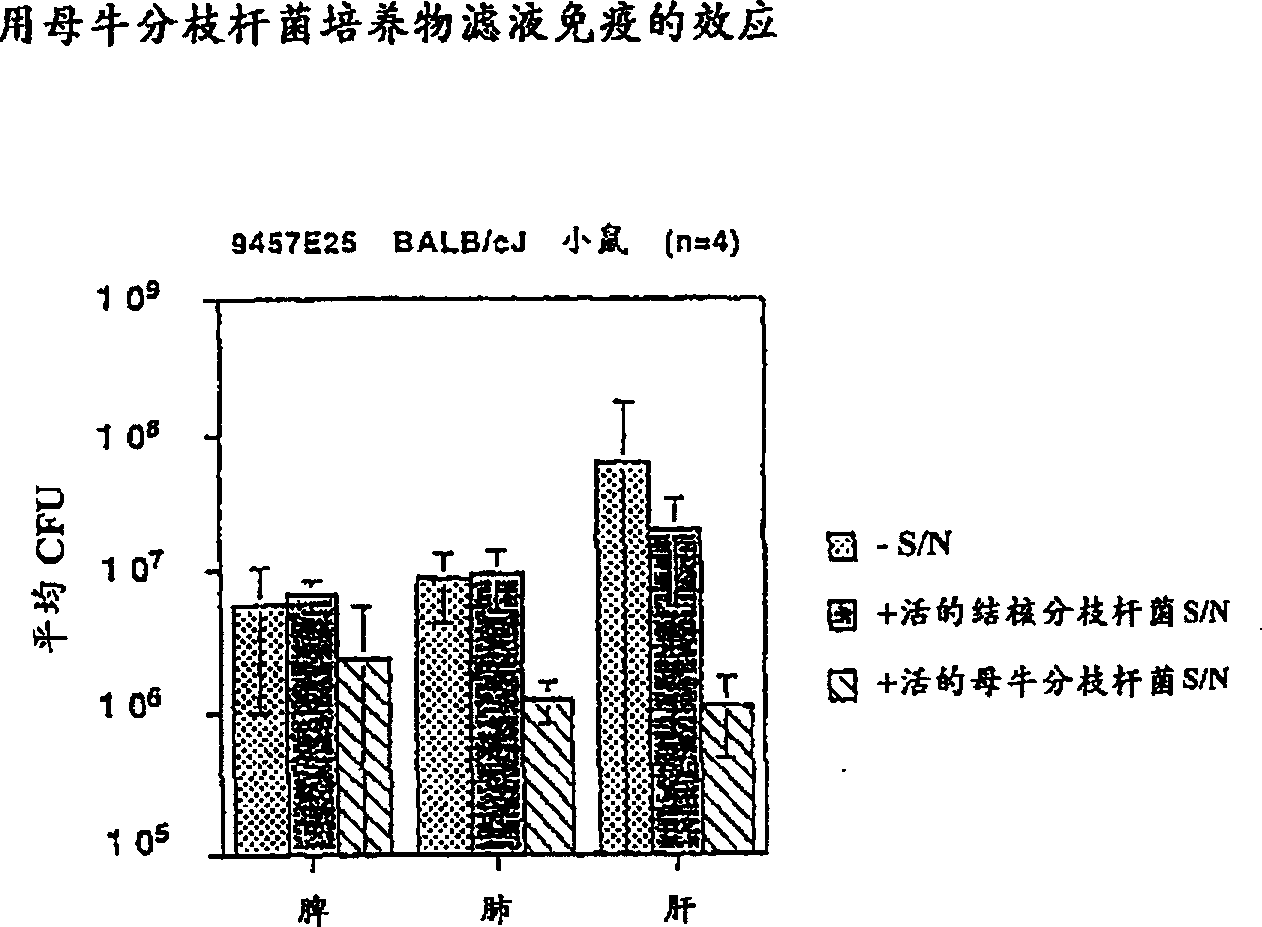
[0139] Immunization with M. vaccae intradermal and intrapulmonary routes
[0140] Effects on macaque tuberculosis
[0141] This example illustrates the effect of immunizing macaques by the intradermal and intrapulmonary routes with heat-killed M. vaccae or M. vaccae culture filtrates prior to challenge with live M. tuberculosis.
[0142]Heat-killed M. vaccae and M. vaccae culture filtrates were prepared as described in Example 1 above. Five groups of macaques were used, with 2 monkeys in each group. Two groups of monkeys were immunized with whole heat-killed M. vaccae either intradermally or intrapulmonarily; two groups of monkeys were immunized with M. vaccae culture filtrate either intradermally or intrapulmonarily; and the control group received no immunization. All immunogens will be dissolved in phosphate buffered saline. Table 1 provides the composition used to immunize each group of monkeys, the amount of immunogen and the route of administration. Before immunizatio...
Embodiment 3
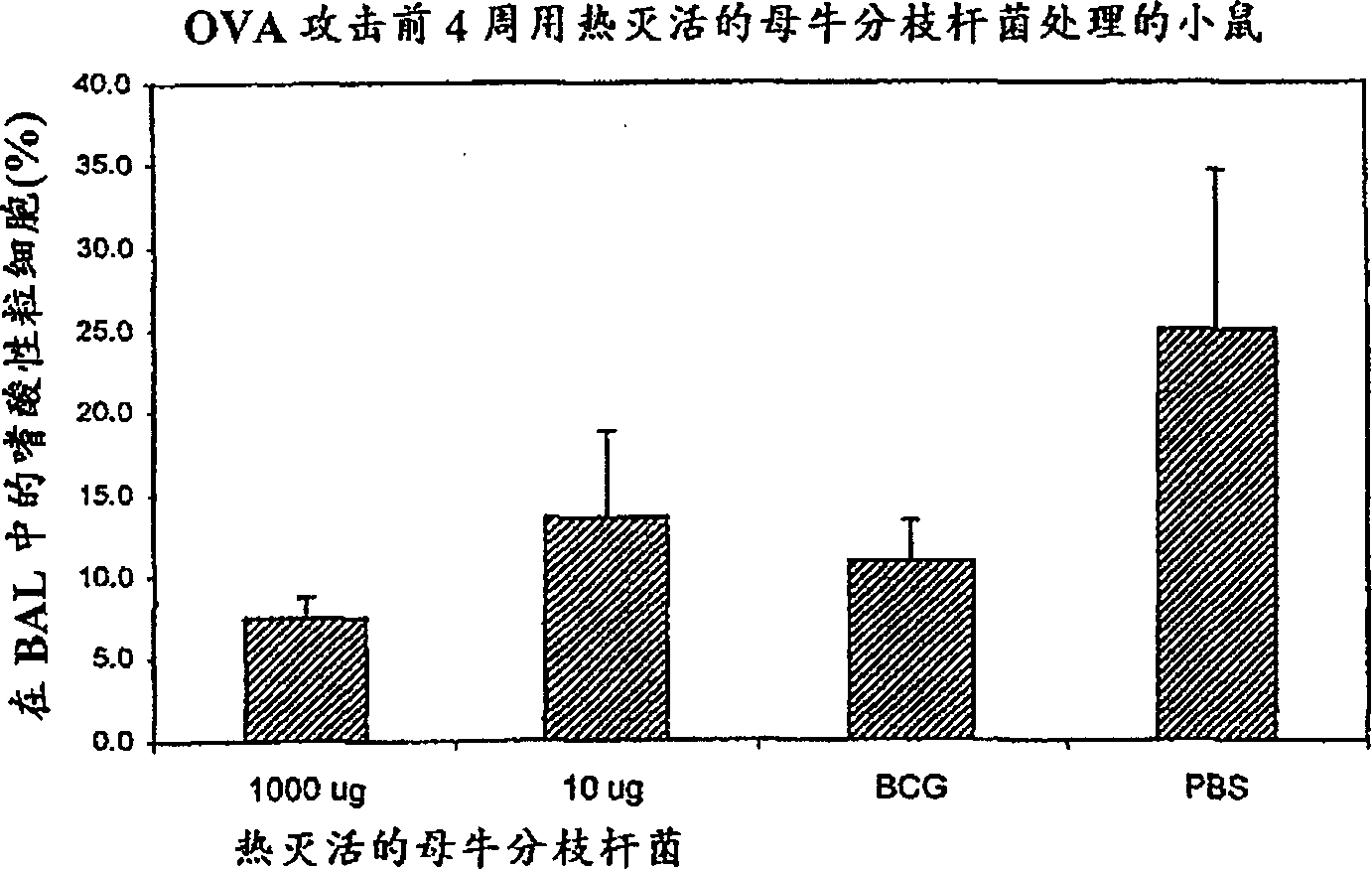
[0156] Effects of Immunization with Mycobacterium vaccae on Asthma in Mice
[0157] This example demonstrates the ability to suppress allergic immune responses in the lungs when heat-killed M. vaccae and DD-M. vaccae were administered intranasally to mice. This was demonstrated in a mouse model of asthma-like allergen-specific lung disease. The severity of this allergic disease is reflected in the large number of eosinophils that accumulate in the lungs.
[0158] C57BL / 6J mice were given 2 μg ovalbumin in 100 μl alum adjuvant by intraperitoneal route at time 0 and 14 days, followed by 100 μg ovalbumin in 50 μl phosphate-buffered saline (PBS) by intranasal route on day 28 protein. It was detected by washing the airways of anesthetized mice with saline, collecting the washings (bronchoalveolar lavage, or BAL), and counting eosinophils, which the mice had accumulated in their lungs.
[0159] Such as Figure 2A and 2B As shown, compared with control mice, 10 or 1000 μg heat-ki...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com