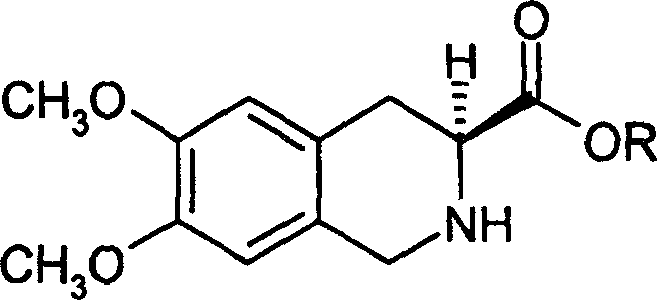
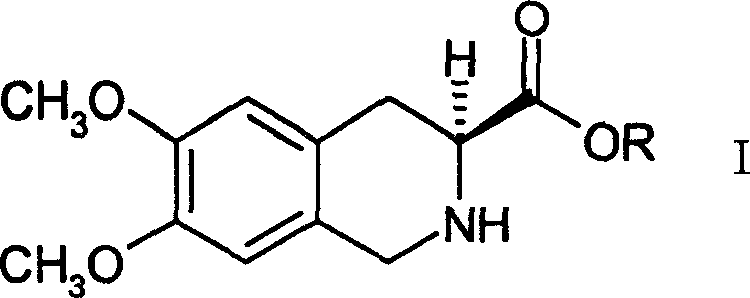
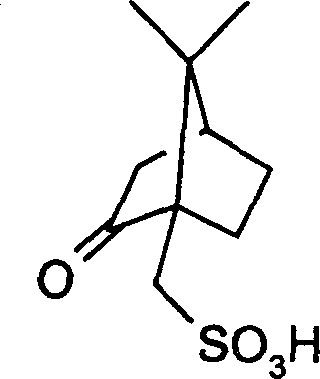
Process for preparing (s)-6,7-dimethoxy-1,2,3,4-tetrahydro isoquinoline-3-carboxylic acid
A technology of tetrahydroisoquinoline and dimethoxy, applied in (S)-6, can solve the problem of low ee value, and achieve the effect of low cost, low equipment and reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of racemic 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolinic acid
[0031] Add 56.2 g of 1,2,3,4-tetrahydro-6,7-dimethoxy-3-isoquinoline hydrochloride to a solution of 25 g of sodium hydroxide and 1,000 ml of water, and stir to dissolve. Add 2 mol / L hydrochloric acid dropwise into the solution, and a white solid precipitates out, continue to add 2 mol / L hydrochloric acid dropwise until pH=6.0, filter to obtain a white crystalline solid. Dry to obtain 64.9 g of (±)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolinic acid.
Embodiment 2
[0033] Preparation of (S)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolinic acid (1R)-10-camphorsulfonate
[0034] Suspend 11.8 g (0.05 mol) of 1,2,3,4-tetrahydro-6,7-dimethoxy-3-isoquinolinic acid in 70 ml of ethanol at room temperature, stir, and (1R)-10 - Pour the solution formed by 11.6 g (0.05 mol) of camphorsulfonic acid and 30 ml of ethanol, and dissolve the solid. Stirring was continued at room temperature, and a white solid was precipitated. After 30 minutes, it was filtered and dried to obtain 11.0 g of a white crystalline solid. Refining (S)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolinic acid (1R)-10-camphorsulfonate with isopropanol (c=1, methanol).
Embodiment 3
[0036] Preparation of (S)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolinic acid
[0037] Dissolve 17.8 g (0.038 mol) of (S)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolinic acid (1R)-10-camphorsulfonate in 270 ml of methanol, Stir, add 3.87g (0.038mol) of triethylamine dropwise at 100°C, a white solid precipitates, continue stirring for 30 minutes, filter, and dry the solid to obtain (S)-6,7-dimethoxy-1,2 , 3,4-tetrahydroisoquinolinic acid 8.4g, (c=1, 1 mol / L HCl).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com