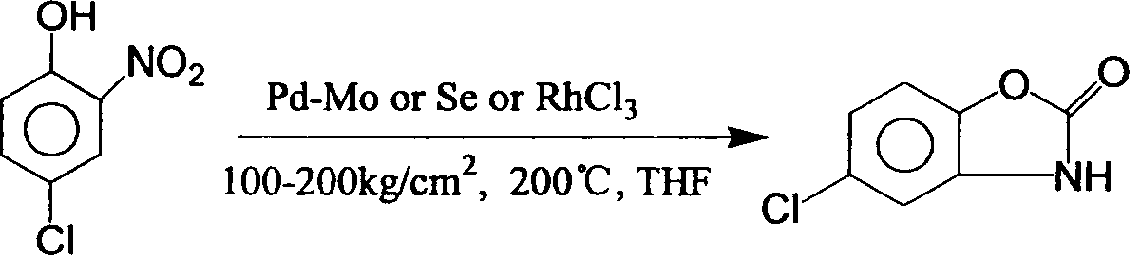Preparation process of chlorazol thazone
A technology for chlorzoxazone and dichloronitrobenzene is applied in the field of preparation of the compound chlorzoxazone, which can solve the problems of unsuitable storage, large human toxicity, difficult application and the like, and achieves the effects of reducing side reactions and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
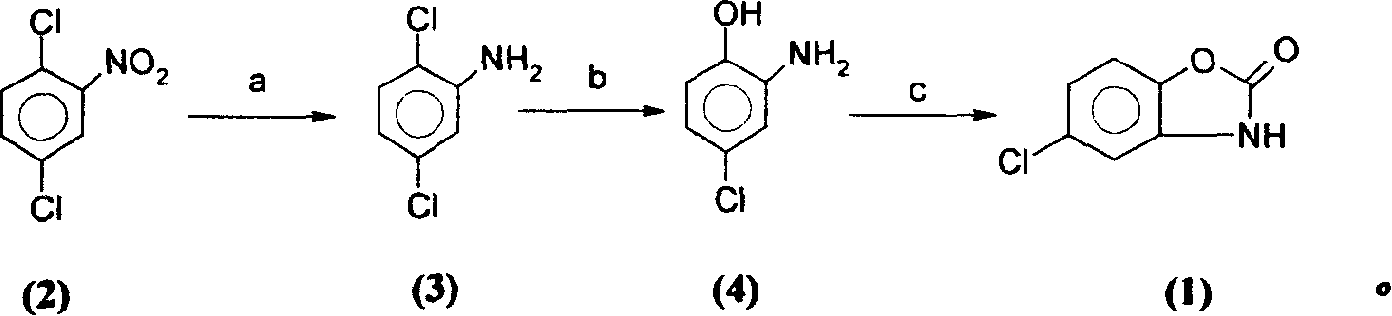
[0028] Example one: the preparation of 2,5-dichloroaniline (3)
[0029] Add 48 grams of compound (2) to 200 milliliters of ethanol, 5 grams of Ranney / Ni, and feed hydrogen under 3Mpa pressure. After 5 hours, the reaction is complete, filter to remove the catalyst, and concentrate to dryness to obtain compound (3), mp=51° C. , yield 91%.
example 2
[0030] Example two: the preparation of 2-amino-4-chlorophenol (4)
[0031] Put 260 grams of compound (3), 650 milliliters of water, and 330 grams of 30% sodium hydroxide into a 2000 milliliter reaction bottle, stir, heat up to 85 ° C, react for 2 hours, cool down to room temperature, add a saturated solution of baking soda to adjust the pH value of the solution Between 8-9, the crude product of compound (4) was obtained by filtration, mp=140°C, yield 86%.
example 3
[0032] Example three: the preparation of chlorzoxazone (1)
[0033] Add 100 grams of compound (4), 180 grams of urea, and 150 grams of 30% hydrochloric acid into a 1000 milliliter reaction flask, heat up to reflux for one hour, add 110 grams of 30% hydrochloric acid and continue the reflux reaction for 30 minutes, then add 100 grams of 30% hydrochloric acid to continue The reaction was refluxed until the reaction was complete (TLC detection), the temperature was lowered to 40° C., and the off-white crude chlorzoxazone was obtained by filtration. Refined with 450 g of 50% ethanol to obtain fine chlorzoxazone (1), mp=191-192°C, yield 85%, and purity greater than 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com