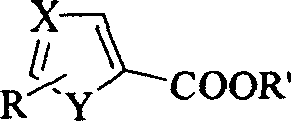
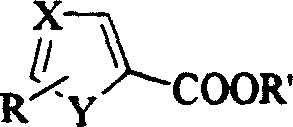
Process for preparing alcohol by heterocyclic carboxylic ester
A technology of heterocyclic carboxylate and heterocycle, which is applied in the field of reduction of aromatic heterocyclic carboxylate to alcohol, which can solve the problems of tetrahydrofuran easy to absorb water, heavy pollution, and inconvenient recycling and treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
[0015] Two (2-methoxyethoxy) sodium aluminum hydride reduction method 1 of embodiment 1.5-thiazole ethyl carboxylate
[0016] Add bis(2-methoxyethoxy)sodium aluminum hydride toluene solution (7.6ml, 7.9g, 27mmol) and 15ml toluene to a dry round bottom flask. Cool the sodium bis(2-methoxyethoxy)aluminum hydride solution to -5~0°C. A solution of ethyl 5-thiazolecarboxylate (3.59 g, 22.85 mmol) and 15 ml of toluene was added dropwise to sodium bis(2-methoxyethoxy)aluminum hydride. Incubate and react at -5~0°C for 1h. The reaction solution was diluted with 75 ml of ethyl acetate. Add 68ml of 15% sodium hydroxide solution to the solution, stir for 30min, extract three times with ethyl acetate, 40ml each time, dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure, and distill under reduced pressure to obtain 5-hydroxymethylthiazole as a yellow liquid 1.63g (98-102°C / 2mmHg), yield 62%. MS(ESI): 116(M+1); 1 H-NMR: 8.737 (s, 1H), 7.701 (s, 1H), 4....
Embodiment 25
[0017] Two (2-methoxyethoxy) sodium aluminum hydride reduction method 2 of embodiment 2.5-thiazole carboxylic acid ethyl ester
[0018] Add bis(2-methoxyethoxy)sodium aluminum hydride toluene solution (152ml, 158g, 540mmol) and 300ml toluene to a dry round bottom flask. Cool the sodium bis(2-methoxyethoxy)aluminum hydride solution to -5~0°C. A solution of ethyl 5-thiazolecarboxylate (71.8 g, 457 mmol) and 300 ml of toluene was added dropwise to sodium bis(2-methoxyethoxy)aluminum hydride. -5 ~ 0 ℃ heat preservation reaction for 1h. The reaction solution was diluted with 1500 ml of ethyl acetate as a solvent. Under stirring, add 22ml of water, 44ml of 10% sodium hydroxide solution and 22ml of water accordingly, stir for 30min, filter with diatomaceous earth, wash with ethyl acetate three times, 200ml each time, dry over anhydrous sodium sulfate, filter, evaporate under reduced pressure solvent. Distillation under reduced pressure gave 37.2 g of yellow liquid 5-hydroxymethyl...
Embodiment 3
[0019] Two (2-methoxyethoxy) sodium aluminum hydride reduction method 3 of embodiment 3.5-thiazole formic acid ethyl ester
[0020] Add bis(2-methoxyethoxy)sodium aluminum hydride toluene solution (38ml, 39.5g, 135mmol) and 60ml of toluene to a dry round bottom flask. Cool the sodium bis(2-methoxyethoxy)aluminum hydride solution to -5~0°C. A solution of ethyl 5-thiazolecarboxylate (7.18g, 45.7mmol) and 30ml of toluene was added dropwise to sodium bis(2-methoxyethoxy)aluminum hydride. -5 ~ 0 ℃ heat preservation reaction for 1h. The reaction solution was diluted with 250 ml of ethyl acetate as a solvent. Under stirring, add 5.5ml of water, 11ml of 10% sodium hydroxide solution and 5.5ml of water accordingly, stir for 30min, filter with diatomaceous earth, wash with ethyl acetate three times, 50ml each time, dry over anhydrous sodium sulfate, filter, and reduce pressure The solvent was evaporated. Distillation under reduced pressure gave 3.78 g of yellow liquid 5-hydroxymethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com