Maskpin colour coupler
A color coupler and general formula technology, applied in multi-color photographic technology, photographic technology, optics, etc., can solve the problem of not fundamentally eliminating harmful absorption, etc., and achieve improved color reproduction quality, increased sensitivity, and high coupling activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
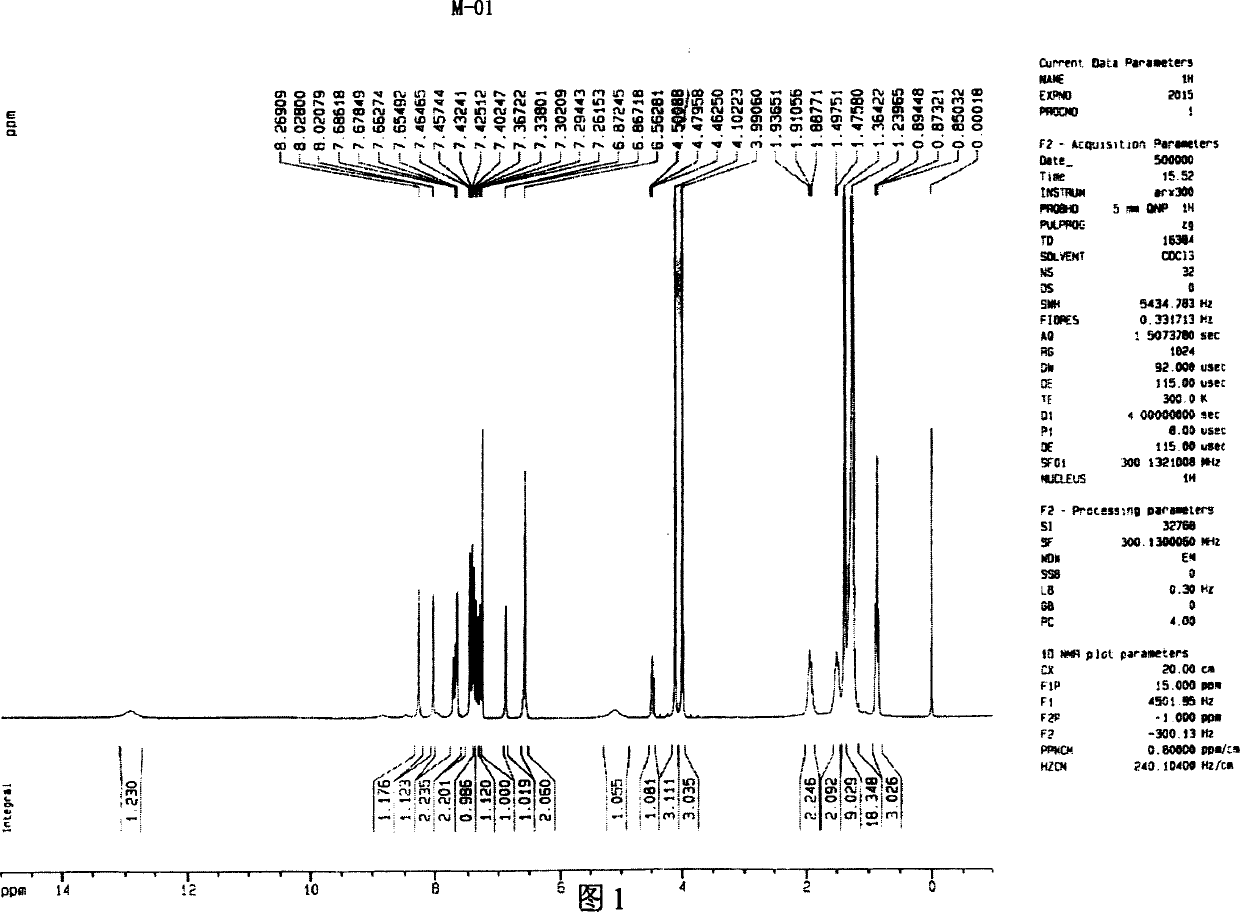
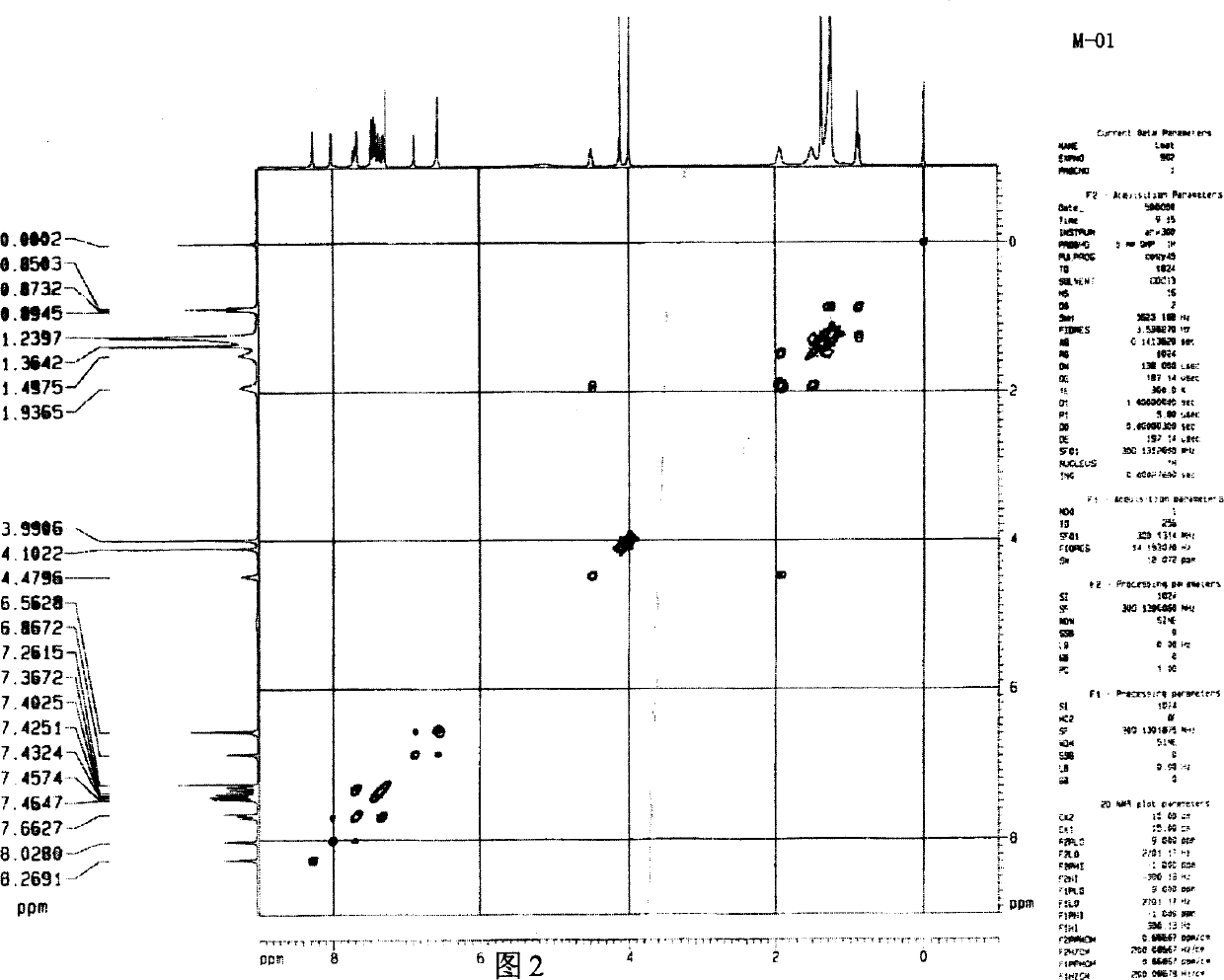
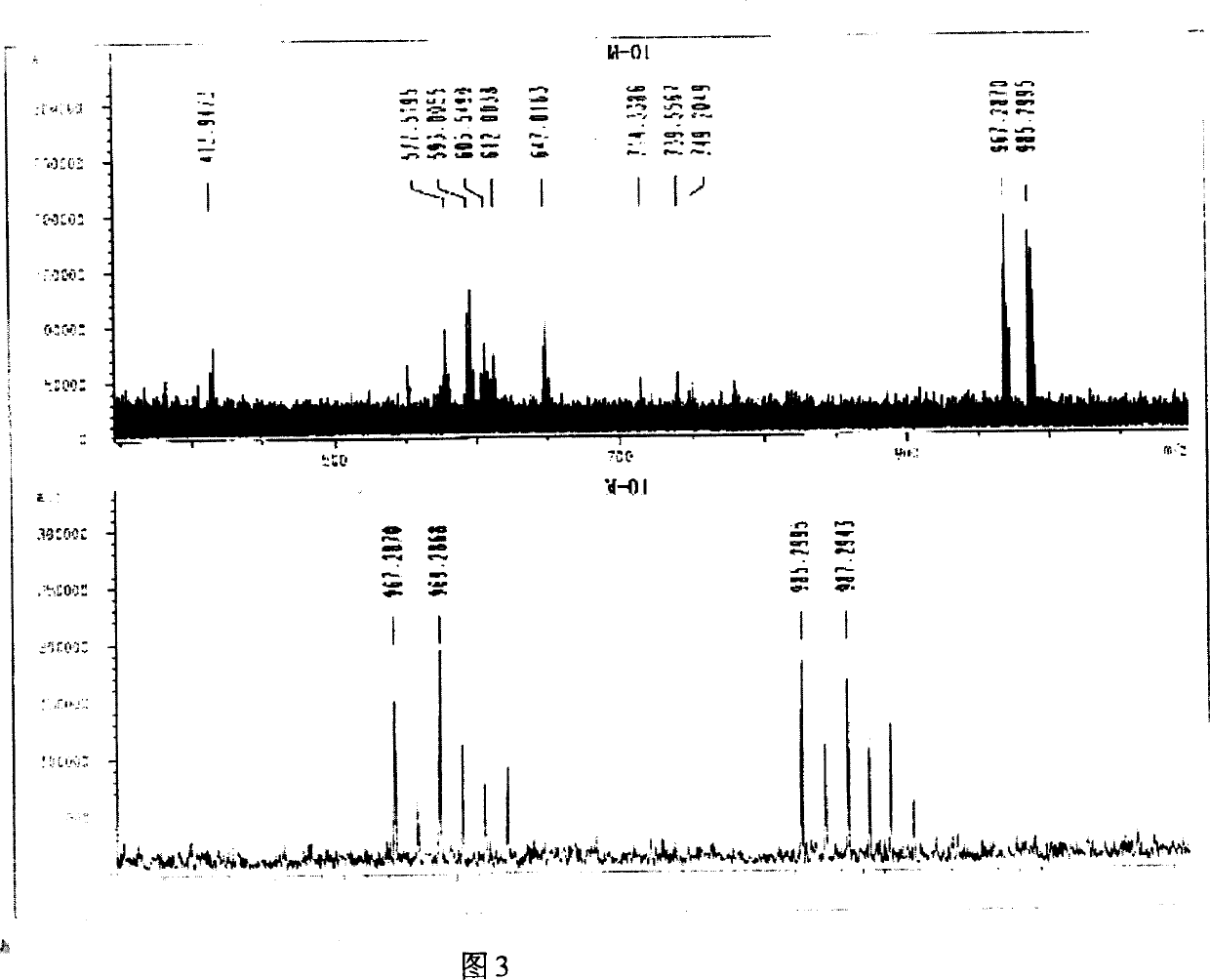
[0037] Embodiment 1: (synthesis of exemplary compound M-01)
[0038] N-[4-chloro-3-[[4-[(3-carboxy-4.5-dimethoxyphenyl)azo]-4.5-dihydro-5-oxo-1-(2.4.6-tri Chlorophenyl)-1H-pyrazol-3-yl]amino]phenyl]-2-[3-(1.1-dimethylethyl)-4-hydroxyphenoxy]-tetradecamide (referred to as M- 01)
[0039] Intermediate 1:
[0040] 2. Synthesis of 3-dimethoxybenzoic acid
[0041] Reaction formula:
[0042]
[0043] Operation method:
[0044] In a 100 ml three-necked flask, add 25 grams of water and 2.5 grams of potassium bicarbonate, and after heating to 95°C, add 2 grams of 2.3-dimethoxybenzaldehyde. Then, 2.5 grams of potassium permanganate solution dissolved in 50 grams of water was added dropwise, and incubated for 2 hours. After cooling to room temperature, it was filtered, and the filtrate was acidified with concentrated hydrochloric acid to pH=1, and a solid precipitated. Filter, wash with water, and dry to obtain 1.7 g of white solid powder, melting point: 122-124°C.
[0045] In...
Embodiment 2
[0064] Embodiment 2: (synthesis of exemplary compound M-17)
[0065] N-[4-chloro-3-[[4-[(4-carboxymethoxyphenyl)azo]-4.5-dihydro-5-oxo-1-(2.4.6-trichlorophenyl) -1H-pyrazol-3-yl]amino]phenyl]-2-[3-(1.1-dimethylethyl)-4-hydroxyphenoxy]-tetradecamide (M-17 for short)
[0066] Intermediate 1:
[0067] Synthesis of p-nitrophenoxyacetic acid
[0068] Reaction formula:
[0069]
[0070] Operation method:
[0071] In a 250 ml three-necked flask, add 110 ml of water and 12.5 g of sodium hydroxide in sequence. After stirring and dissolving, 21 grams of p-nitrophenol was added, and a mixed solution of 15 grams of chloroacetic acid and 35 milliliters of water was added dropwise. Then, the temperature was raised to reflux, and after insulation for 3 hours, a mixed solution of 7.5 grams of chloroacetic acid, 6.5 grams of sodium hydroxide and 30 milliliters of water was added dropwise, and the reflux reaction was continued for 6 hours. Then, it was cooled to room temperature, acidi...
Embodiment 3
[0085] Embodiment 3: (synthesis of exemplary compound M-19)
[0086] N-[4-chloro-3-[[4-[(4-hydroxyethoxyphenyl)azo]-4.5-dihydro-5-oxo-1-(2.4.6-trichlorophenyl) -1H-pyrazol-3-yl]amino]phenyl]-2-[3-(1.1-dimethylethyl)-4-hydroxyphenoxy]-tetradecamide (M-19 for short)
[0087] Intermediate 1:
[0088] Synthesis of p-Nitrophenoxyethanol
[0089] Reaction formula:
[0090]
[0091] Operation method:
[0092] In a 100 ml three-necked flask, 75 ml of water, 2.6 g of sodium hydroxide, and 8.8 g of p-nitrophenol were successively added. The temperature was raised to 80° C., 5.6 g of chloroethanol was added dropwise, and then refluxed for 30 hours. After cooling to room temperature, filter, wash with water, and dry to obtain 5 grams of off-white powder with a melting point of 82-83°C.
[0093] Intermediate 2:
[0094] Synthesis of p-aminophenoxyethanol
[0095] Reaction formula:
[0096]
[0097] Operation method:
[0098] In a 100ML three-necked flask, add 4.0 grams of p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com