Ivermectin slow-release injecta, and preparing process and use method thereof
An injection preparation and ivermectin technology, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of no long-term continuous insecticidal effect, short drug effect time, etc., and achieve the overcoming effect. The effect of short time, low toxic and side effects, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
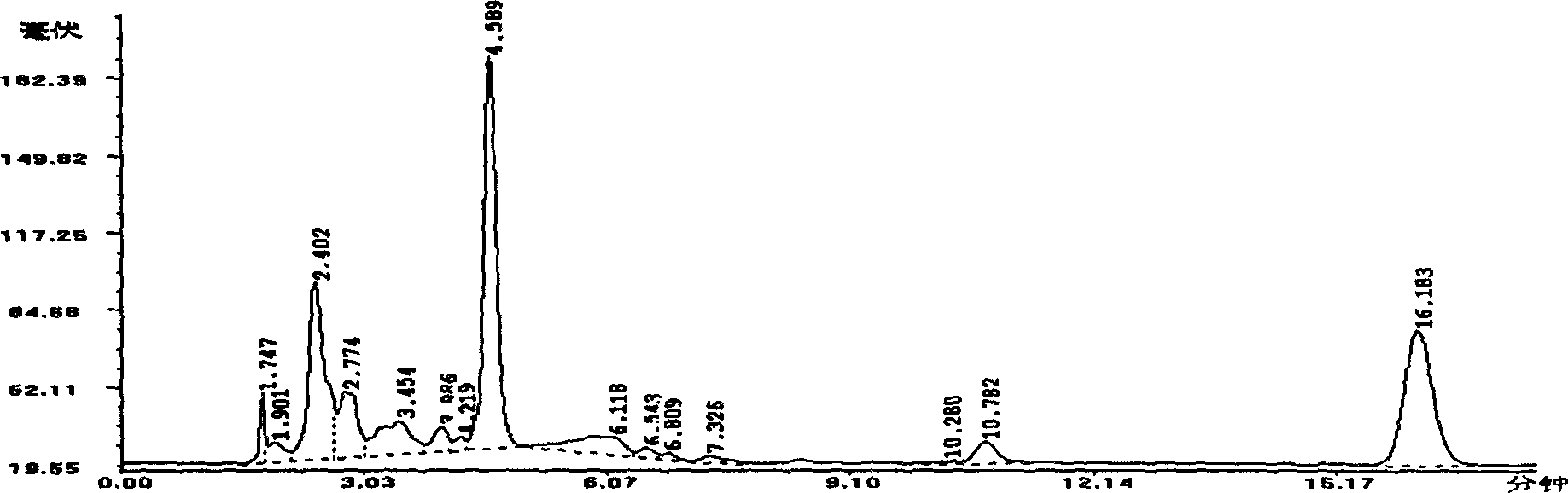
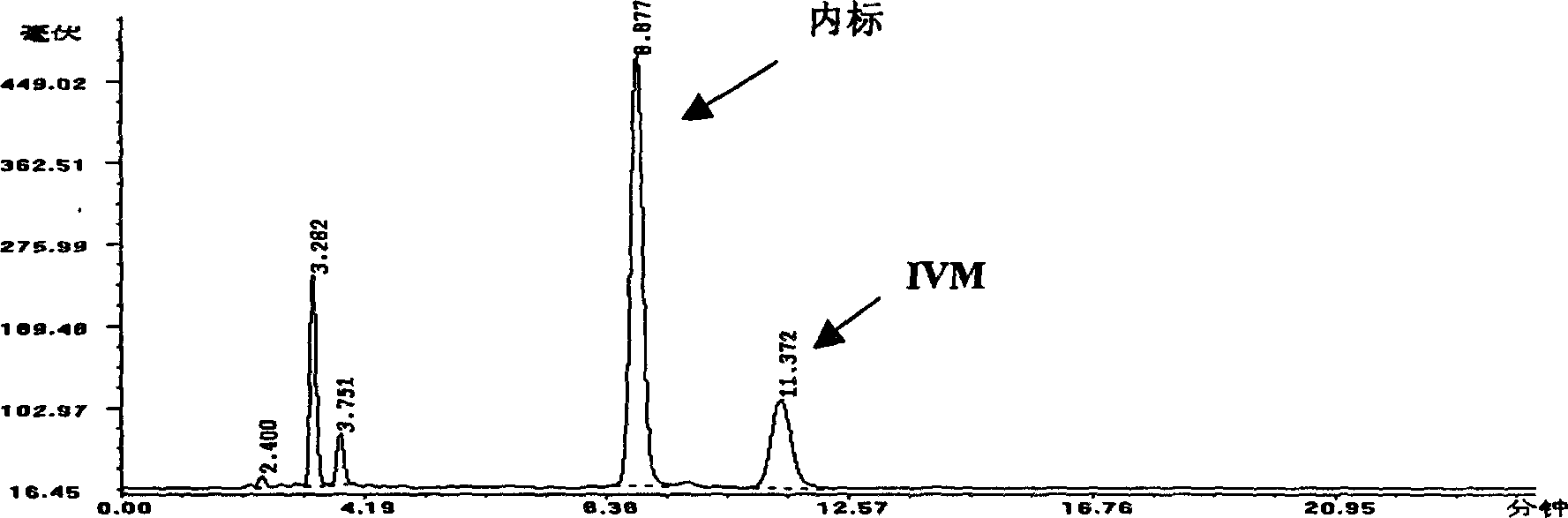
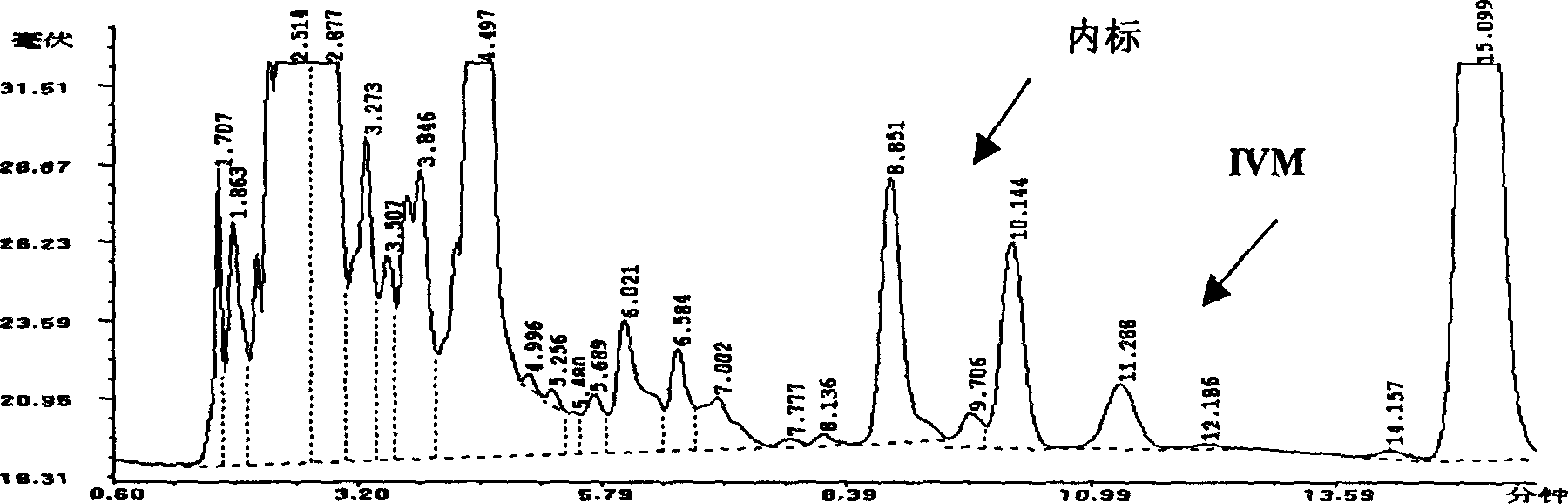
Image
Examples
Embodiment 1
[0028] Example 1, the ivermectin sustained-release injection contains by weight percentage: 5% ivermectin, 5% ethyl acetate, 1% aluminum monostearate and the rest castor oil for injection.
Embodiment 2
[0029] Example 2, the ivermectin sustained-release injection contains by weight percentage: 5% ivermectin, 15% ethyl acetate, 5% aluminum monostearate and the rest castor oil for injection.
Embodiment 3
[0030] Example 3, the ivermectin sustained-release injection contains by weight percentage: 5% ivermectin, 5% ethyl acetate, 5% aluminum monostearate and the rest castor oil for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com